Abstract
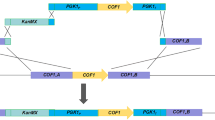
Proteinase A (PrA), encoded by PEP4 gene, is detrimental to beer foam stability. There are two transport pathways for the new synthesized PrA in yeast, sorting to the vacuole normally, or excreting out of the cells under stress conditions. They were designated as the Golgi-to-vacuole pathway and the constitutive secretory pathway, respectively. To reduce PrA excretion in some new way instead of its coding gene deletion, which had a negative effect on cell metabolism and beer fermentation, we modified the PrA transport based on these above two pathways. In the Golgi-to-vacuole pathway, after the verification that Vps10p is the dominant sorting receptor for PrA Golgi-to-vacuolar transportation by VPS10 deletion, VPS10 was then overexpressed. Furthermore, SEC5, encoding exocyst complexes’ central subunit (Sec5p) in the constitutive secretory pathway, was deleted. The results show that PrA activity in the broth fermented with WGV10 (VPS10 overexpressing strain) and W∆SEC5 (SEC5 deletion strain) was lowered by 76.96 and 32.39%, compared with the parental strain W303-1A, at the end of main fermentation. There are negligible changes in fermentation performance between W∆SEC5 and W303-1A, whereas, surprisingly, WGV10 had a significantly improved fermentation performance compared with W303-1A. WGV10 has an increased growth rate, resulting in higher biomass and faster fermentation speed; finally, wort fermentation is performed thoroughly. The results show that the biomass production of WGV10 is always higher than that of W∆SEC5 and W303-1A at all stages of fermentation, and that ethanol production of WGV10 is 1.41-fold higher than that of W303-1A. Obviously, VPS10 overexpression is beneficial for yeast and is a more promising method for reduction of PrA excretion.






Similar content being viewed by others
References
Ammerer G, Hunter CP, Rothman JH, Saari GC, Valls LA, Stevens TH (1986) PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol 6:2490–2499. doi:10.1128/MCB.6.7.2490
Rendueles PS, Wolf DH (1988) Proteinase function in yeast: biochemical and genetic approaches to a central mechanism of post-translational control in the eukaryote cell. FEMS Microbiol Rev 54:17–46. doi:10.1111/j.1574-6968.1988.tb02706.x-i1
Westphal V, Marcusson EG, Winther JR, Emr SD, van den Hazel HB (1996) Multiple pathways for vacuolar sorting of yeast proteinase A. J Biol Chem 271:11865–11870. doi:10.1074/jbc.271.20.11865
Kondo H, Yomo H, Furukubo S, Fukui N, Nakatani K, Kawasaki Y (1999) Advanced method for measuring proteinase A in beer and application to brewing. J Inst Brew 105:293–300. doi:10.1002/j.2050-0416.1999.tb00523.x
Lu J, Dong J, Wu D, Chen Y, Guo X, Shi Y, Sun X, Xiao D (2012) Construction of recombinant industrial brewer’s yeast with lower diacetyl production and proteinase A activity. Eur Food Res Technol 235:951–961. doi:10.1007/s00217-012-1821-9
Wang ZY, He GQ, Liu ZS, Ruan H, Chen QH, Xiong HP (2005) Purification of yeast proteinase A from fresh beer and its specificity on foam proteins. Int J Food Sci Technol 40:835–840. doi:10.1111/j.1365-2621.2005.01000.x
Fukal L, Káš J, Rauch P (1986) Properties of yeast proteinases. J Inst Brew 92:357–359. doi:10.1002/j.2050-0416.1986.tb04423.x
He GQ, Wang ZY, Liu ZS, Chen QH, Ruan H, Schwarz PB (2006) Relationship of proteinase activity, foam proteins, and head retention in unpasteurized beer. J Am Soc Brew Chem 64:33–38
Zhang HB, Ruan H, Li WF, Zhang W, Su ZR, He GQ, Chen QH (2011) Construction of recombinant industrial S. cerevisiae strain with barley lipid-transfer protein 1 secretion capability and lower PrA activity. Eur Food Res Technol 233:707–716. doi:10.1007/s00217-011-1559-9
Cooper DJ, Stewart GG, Bryce JH (2000) Yeast proteolytic activity during high and low gravity wort fermentations and its effect on head retention. J Inst Brew 106:197–201. doi:10.1002/j.2050-0416.2000.tb00057.x
Liu C, Song Q, Yin X (2014) Effect of yeast physiological indexes on proteinase A secretion detected by resonance light scattering technique. Eur Food Res Technol 239:913–921. doi:10.1007/s00217-014-2288-7
Hao J, Dong J, Speers RA, Shen W, Shan L, Fan W, Li Q, Gu G, Chen J (2008) Construction of a single PEP4 allele deletion in Saccharomyces carlsbergensis and a preliminary evaluation of its brewing performance. J Inst Brew 114:322–328. doi:10.1002/j.2050-0416.2008.tb00776.x
Liu XF, Wang ZY, Wang JJ, Lu Y, He XP, Zhang BR (2009) Expression of GAI gene and disruption of PEP4 gene in an industrial brewer’s yeast strain. Lett Appl Microbiol 49:117–123. doi:10.1111/j.1472-765X.2009.02627.x
Wang ZY, He GQ, Ruan H, Liu ZS, Yang LF, Zhang BR (2007) Construction of proteinase A deficient transformant of industrial brewing yeast. Eur Food Res Technol 225:831–835. doi:10.1007/s00217-006-0488-5
Wang ZY, He XP, Zhang BR (2007) Over-expression of GSH1 gene and disruption of PEP4 gene in self-cloning industrial brewer’s yeast. Int J Food Microbiol 119:192–199. doi:10.1016/j.ijfoodmicro.2007.07.015
Kato M, Kuzuhara Y, Maeda H, Shiraga S, Ueda M (2006) Analysis of a processing system for proteases using yeast cell surface engineering: conversion of precursor of proteinase A to active proteinase A. Appl Microbiol Biotechnol 72:1229–1237. doi:10.1007/s00253-006-0408-4
Parr CL, Keates RAB, Bryksa BC, Ogawa M, Yada RY (2007) The structure and function of Saccharomyces cerevisiae proteinase A. Yeast 24:467–480. doi:10.1002/yea.1485
Rupp S, Hirsch HH, Wolf DH (1991) Biogenesis of the yeast vacuole (lysosome). Active site mutation in the vacuolar aspartate proteinase yscA blocks maturation of vacuolar proteinases. FEBS Lett 293:62–66. doi:10.1016/0014-5793(91)81153-Y
Chen Qh, Liu XJ, Fu ML, Zhang HB (2010) Effect of PrA encoding gene-PEP4 deletion in industrial S. cerevisiae WZ65 on key enzymes in relation to the glycolytic pathway. Eur Food Res Technol 231:943–950. doi:10.1007/s00217-010-1355-y
Liu XJ, Feng Y, Fu ML, Dong YC, Chen QH, Jiao YC (2011) The shock of vacuolar PrA on glycolytic flux oxidative phosphorylation and cell morphology by industrial Saccharomyces cerevisiae WZ65. Eur Food Res Technol 233:941–949. doi:10.1007/s00217-011-1586-6
Bröcker C, Engelbrecht-Vandré S, Ungermann C (2010) Multisubunit tethering complexes and their role in membrane fusion. Curr Biol 20:944–952. doi:10.1016/j.cub.2010.09.015
Mechler B, Müller M, Müller H, Meussdoerffer F, Wolf DH (1982) In vivo biosynthesis of the vacuolar proteinases A and B in the yeast Saccharomyces cerevisiae. J Biol Chem 257:11203–11206
Klionsky DJ, Banta LM, Emr SD (1988) Intracellular sorting and processing of a yeast vacuolar hydrolase: proteinase A propeptide contains vacuolar targeting information. Mol Cell Biol 8:2105–2116. doi:10.1128/MCB.8.5.2105
Stevens T, Esmon B, Scheckman R (1982) Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell 30:439–448. doi:10.1016/0092-8674(82)90241-0
Cooper AA, Stevens TH (1996) Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J Cell Biol 133:529–541. doi:10.1083/jcb.133.3.529
Meussdoerffer F, Tortora P, Holzer H (1980) Purification and properties of proteinase A from yeast. J Biol Chem 255:12087–12093
Van den Hazel HB, Wolff AM, Kielland-Brandt MC, Winther JR (1997) Mechanism and ion-dependence of in vitro autoactivation of yeast proteinase A: possible implications for compartmentalized activation in vivo. J Biochem 326:339–344. doi:10.1042/bj3260339
Wolff AM, Din N, Petersen JGL (1996) Vacuolar and extracellular maturation of Saccharomyces cerevisiae proteinase A. Yeast 12:823–832. doi:10.1002/(SICI)1097-0061(199607)12:9<823:AID-YEA975>3.0.CO;2-J
Rothman JH, Hunter CP, Valls LA, Stevens TH (1986) Overproduction-induced mislocalization of a yeast vacuolar protein allows isolation of its structural gene. Proc Nati Acad Sci USA 83:3248–3252
JØrgensen MU, Emr SD, Winther JR (1999) Ligand recognition and domain structure of Vps10p a vacuolar protein sorting receptor in Saccharomyces cerevisiae. Eur J Biochem 260:461–469. doi:10.1046/j.1432-1327.1999.00176.x
Whyte JRC, Munro S (2001) A yeast homolog of the mammalian mannose 6-phosphate receptors contributes to the sorting of vacuolar hydrolases. Curr Biol 11:1074–1078. doi:10.1016/S0960-9822(01)00273-1
Bednarek SY, Wilkins TA, Dombrowski JE, Raikhel NV (1990) A carboxyl-terminal propeptide is necessary for proper sorting of barley lectin to vacuoles of tobacco. Plant Cell 2:1145–1155. doi:10.1105/tpc.2.12.1145
Stack JH, Emr SD (1993) Genetic and biochemical studies of protein sorting to the yeast vacuole. Curr Opin Cell Biol 5:641–646. doi:10.1016/0955-0674(93)90134-C
Salminen A, Novick PJ (1989) The Sec15 protein responds to the function of the GTP binding protein Sec4 to control vesicular traffic in yeast. J Cell Biol 109:1023–1036. doi:10.1083/jcb.109.3.1023
Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116:153–166. doi:10.1016/S0092-8674(03)01079-1
Liu J, Guo W (2012) The exocyst complex in exocytosis and cell migration. Protoplasma 249:587–597. doi:10.1007/s00709-011-0330-1
Daniel RT, Peter N (1995) Sec6, Sec8, and Secl5 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. EMBO J 130:299–312. doi:10.1083/jcb.130.2.299
Zhu B, Cai G, Hall EO, Freeman GJ (2007) In-fusion™ assembly: seamless engineering of multidomain fusion proteins, modular vectors, and mutations. Biotechniques 43:354–359. doi:10.2144/000112536
Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH (2002) A second set of loxP marker cassettes for cre-mediated multiple gene knockouts in budding yeast. Nucl Acid Res 30:e23. doi:10.1093/nar/30.6.e23
Hsu SC, TerBush D, Abraham M, Guo W (2004) The exocyst complex in polarized exocytosis. Int Rev Cytol 233:243–265. doi:10.1016/S0074-7696(04)33006-8
Liu JL, Guo W (2012) The exocyst complex in exocytosis and cell migration. Protoplasma 249:587–597. doi:10.1007/s00709-011-0330-1
Conibear E, Stevens TH (1998) Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim Biophys Acta 1440:211–230. doi:10.1016/S0167-4889(98)00058-5
Mala M, Rita OT, Tamara PM, Thomas LS (2010) Sec5 a member of the exocyst complex mediates drosophila embryo cellularization. Development 141:2773–2783. doi:10.1242/dev.048330
Wan K, Yabuki Y, Mizuta K (2015) Roles of Ebp2 and ribosomal protein L36 in ribosome biogenesis in Saccharomyces cerevisiae. Curr Genet 61:31–41. doi:10.1007/s00294-014-0442-1
Lilly M, Lambrechts MG, Pretorius IS (2000) Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl Environ Microbiol 66:744–753. doi:10.1128/AEM.66.2.744-753.2000
Acknowledgements
The current study was financially supported by the program for the National Natural Science Foundation of China (No. 31271916).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chen, Y., Song, L., Han, Y. et al. Decreased proteinase A excretion by strengthening its vacuolar sorting and weakening its constitutive secretion in Saccharomyces cerevisiae . J Ind Microbiol Biotechnol 44, 149–159 (2017). https://doi.org/10.1007/s10295-016-1868-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1868-x