Abstract
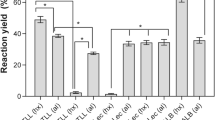
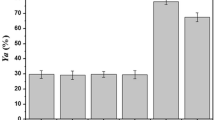
A partially purified lipase produced by the thermophile Geobacillus thermoleovorans CCR11 was immobilized by adsorption on porous polypropylene (Accurel EP-100) in the presence and absence of 0.1% Triton X-100. Lipase production was induced in a 2.5% high oleic safflower oil medium and the enzyme was partially purified by diafiltration (co. 500,000 Da). Immobilization conditions were established at 25 °C, pH 6, and a protein concentration of 0.9 mg/mL in the presence and absence of 0.1% Triton X-100. Immobilization increased enzyme thermostability but there was no change in neither the optimum pH nor in pH resistance irrelevant to the presence of the detergent during immobilization. Immobilization with or without Triton X-100 allowed the reuse of the lipase preparation for 11 and 8 cycles, respectively. There was a significant difference between residual activity of immobilized and soluble enzyme after 36 days of storage at 4 °C (P < 0.05). With respect to chain length specificity, the immobilized lipase showed less activity over short chain esters than the soluble lipase. The immobilized lipase showed good resistance to desorption with phosphate buffer and NaCl; minor loses with detergents were observed (less than 50% with Triton X-100 and Tween-80), but activity was completely lost with SDS. Immobilization of G. thermoleovorans CCR11 lipase in porous polypropylene is a simple and easy method to obtain a biocatalyst with increased stability, improved performance, with the possibility for re-use, and therefore an interesting potential use in commercial conditions.





Similar content being viewed by others
References
Jaeger KE, Dijkstra BW, Reetz MT (1999) Bacterial biocatalysts: molecular biology, three-dimensional structures and biotechnological applications of lipases. Annu Rev Microbiol 53:315–351. doi:10.1146/annurev.micro.53.1.315
Sharma R, Chisti Y, Banerjee UC (2001) Production, purification, characterization and applications of lipases. Biotechnol Adv 19:627–662. doi:10.1016/S0734-9750(01)00086-6
Minovska V, Winkelhausen E, Kuzmanova S (2005) Lipases immobilized by different techniques on various support materials applied in oil hydrolysis. J Serb Chem Soc 70:609–624. doi:10.2298/JSC0504609M
Leow TC, Rahman RN, Basri M, Salleh AB (2004) High level expression of a thermostable lipase from Geobacillus sp. Strain T1. Biosci Biotechnol Biochem 68(1):96–103. doi:10.1271/bbb.68.96
Hill CG Jr, Otero C, García HS (2006) Immobilized Enzyme Technology. In: Encyclopedia of Chemical Processing. Tyler and Francis, pp 1367–1380
Tischer W, Wedekind F (1999) Immobilized enzymes: methods and applications. Top Curr Chem 200:96–126
Malcata FX, Reyes HR, García HS, Hill CG, Amundson CH (1990) Immobilized lipase reactors for modification of fats and oils: a review. J Am Oil Chem Soc 67:890–910. doi:10.1007/BF02541845
Malcata FX, García HS, Hill CG Jr, Amundson CH (1992) Hydrolysis of butteroil by immobilized lipase using a hollow-fiber reactor: Part I lipase adsorption studies. Biotechnol Bioeng 39:647–657. doi:10.1002/bit.260390609
García HS, Yang B, Parkin KL (1996) Continuous reactor for enzymatic glycerolisis of butteroil in the absence of solvent. Food Res Int 28:605–609. doi:10.1016/0963-9969(95)00051-8
Pencreac’h G, Baratti JC (1997) Activity of Pseudomonas cepacia in organic media is greatly enhanced after immobilization on a polypropylene support. Appl Microbiol Biotechnol 47:630–635. doi:10.1007/s002530050986
Pencreac’h G, Leullier M, Baratti JC (1997) Properties of free and immobilized lipase from Pseudomonas cepacia. Biotechnol Bioeng 56:181–189. doi :10.1002/(SICI)1097-0290(19971020)56:2<;181::AID-BIT7>;3.0.CO;2-L
Ferrer M, Plou FJ, Fuentes G, Cruces MA, Andersen L, Kirk O et al (2002) Effect of the immobilization method of lipase from Thermomyces lanuginosus on sucrose acylation. Biocatal Biotransform 20:63–71. doi:10.1080/10242420210153
Deng HT, Xu ZK, Huang XJ, Wu J, Seta P (2004) Adsorption and activity of Candida rugosa lipase on polypropylene hollow fiber membrane modified with phospholipid analogous polymers. Langmuir 20:10168–10173. doi:10.1021/la0484624
Bano MC, González-Navarro H, Abad C (2003) Long chain fatty acyl-CoA esters induce lipase activation in the absence of a water-lipid interface. Biochem Biophys A 1632:55–61
Castro-Ochoa LD, Rodríguez-Gómez C, Valerio-Alfaro G, Oliart-Ros RM (2005) Screening purification and characterization of the thermoalkalophilic lipase produced by Bacillus thermoleovorans CCR11. Enzyme Microb Technol 37:648–654. doi:10.1016/j.enzmictec.2005.06.003
Fernández-Lorente G, Palomo JM, Cabrera Z, Fernández-Lafuente R, Guisán JM (2007) Improved catalytic properties of immobilized lipases by the presence of very low concentrations of detergents in the reaction medium. Biotechnol Bioeng 97:242–250. doi:10.1002/bit.21230
Helisto P, Korpela T (1998) Effects of detergents on activity of microbial lipases as measured by nitrophenyl alkanoate esters method. Enzyme Microb Technol 23:113–117. doi:10.1016/S0141-0229(98)00024-6
Rodrigues AR, Cabral JMS, Taipa MA (2002) Immobilization of Chromobacterium viscosum lipase on Eudragit S-100: coupling, characterization and kinetic application in organic and biphasic media. Enzyme Microb Technol 31:133–141. doi:10.1016/S0141-0229(02)00087-X
Palomo JM, Fuentes M, Fernández-Lorente G, Mateo C, Guisán JM, Fernández-Lafuente R (2003) General trend of lipase to self-assemble giving biomolecular aggregates greatly modifies the enzyme functionality. Biomacromol 4:1–6. doi:10.1021/bm025729+
Palomo JM, Segura RL, Fernández-Lorente G, Pernas M, Rua ML, Guisán JM et al (2004) Purification, immobilization and stabilization of a lipase from Bacillus thermocatenulatus by interfacial adsorption on hydrophobic supports. Biotechnol Prog 20:630–635. doi:10.1021/bp0342957
Nawani N, Dosanjh NS, Kaur J (1988) A novel thermostable lipase from a thermophilic Bacillus sp: characterization and esterification studies. Biotechnol Lett 20:997–1000. doi:10.1023/A:1005430215849
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1955) Protein measurement with the Folin phenol reagent. Department of Pharmacology, Washington University. School of Medicine, St. Louis, Missouri, pp 265–275
Bosley JA, Peilow AD (1997) Immobilization of lipases on porous polypropylene: reduction in esterification efficiency at low loading. J Am Oil Chem Soc 74:107–111. doi:10.1007/s11746-997-0153-6
Bastida A, Sabuquillo P, Armisen P, Fernández-Lafuente R, Huguet J, Guisán JM (1998) A single step purification immobilization and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol Bioeng 58:486–493. doi :10.1002/(SICI)1097-0290(19980605)58:5<;486::AID-BIT4>;3.0.CO;2-9
Dosanjh NS, Kaur J (2002) Immobilization stability and esterification studies of a lipase from Bacillus sp. Biotechnol Appl Biochem 36:7–12. doi:10.1042/BA20010070
Fernández-Lafuente R, Armisén P, Sabuquillo P, Fernández-Lorente G, Guisán JM (1998) Immobilization of lipases by selective adsorption on hydrophobic supports. Chem Phys Lipids 93:185–197. doi:10.1016/S0009-3084(98)00042-5
Acknowledgments
M. Sc Guadalupe Sánchez Otero acknowledges her scholarship from the Mexican National Council for Science and Technology (Conacyt). This work was supported by grant UR-612-04 from the General Direction of Superior Technological Studies (DGEST-SEP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez-Otero, M.G., Valerio-Alfaro, G., García-Galindo, H.S. et al. Immobilization in the presence of Triton X-100: modifications in activity and thermostability of Geobacillus thermoleovorans CCR11 lipase. J Ind Microbiol Biotechnol 35, 1687–1693 (2008). https://doi.org/10.1007/s10295-008-0433-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0433-7