Abstract
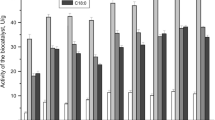
Rhizomucor miehei, Humicola sp., Rhizopus niveus, and Candida antarctica B lipases were immobilized by physical adsorption onto a macroporous polypropylene support. In an esterification reaction, the enzyme efficiency, and therefore cost-effectiveness, is greatly affected by enzyme loading, with an apparent suppression of efficiency at low lipase loadings for both R. miehei and Humicola sp. lipases. This results in the appearance of a pronounced maximum in the efficiency-loading relationship at approximately 100,000 lipase units (LU)/g for R. miehei lipase (10% of its saturation loading) and at approximately 200,000 LU/g for Humicola sp. lipase (50% of its saturation loading). The other lipases studied do not show similar trends. At low loadings, only a small portion of the surface area is occupied and gives the lipase the opportunity to spread; it is hypothesized that the reduction in efficiency at low loadings is due to a distortion of the active molecular conformation caused by the lipase maximizing its contact with the support as a result of its high affinityfor the support surface. The relationship between efficiency and loading was different for each of the lipases studied, which may reflect both differences in the strength of the affinity of the lipase for the support and in the ease at which the molecular conformation of the lipase can be distorted.
Similar content being viewed by others
References
Macrae, A.R., and R.C. Hammond, Present and Future Applications of Lipases, Biotechnol. Gen. Eng. Rev. 3:193–217 (1985).
Bjorkling, F., S.E. Godtfredson, and O. Kirk, The Future Impact of Industrial Lipases, TIBTECH 9:360–363 (1991).
Eigtved, P., Enzymes and Lipid Modification, in Advances in Applied Lipid Research (edited by F.B. Padley), JAI Press, London, Vol. 1, 1992, pp. 1–64.
Quinlan, P., and S. Moore, Modification of Triglycerides by Lipases: Process Technology and Its Application to the Production of Nutritionally Improved Fats, INFORM 4:580–585 (1993).
Macrae, A.R., E.L. Roehl, and H.M. Brand, Bio-esters, Seifen Öle Fette Wasche 116:201–205 (1984).
Wisdom, R.A., P. Dunnill, M.D. Lilly, and A.R. Macrae, Enzyme Esterification of Fats: Factors Influencing the Choice of Support for Immobilized Lipase, Enzyme Microb. Technol. 6:443–446 (1984).
Malcata, F.X., H.R. Reyes, H.S. Garcia, C.G. Hill Jr., and C.H. Amundson, Immobilized Lipase Reactors for Modification of Fats and Oil—A Review, J. Am. Oil Chem. Soc. 67:890–910 (1990).
Eigtved, P., An Immobilized Lipase Preparation and Its Use Thereof, European Patent Application EP 140,542 (1985).
Ibrahim, C.O., H. Saeki, N. Nishio, and S. Nagai, Hydrolysis of Triglycerides by Immobilized Thermostable Lipase from Humicola lanuginosa, Agric. Biol. Chem. 52:99–105 (1988).
Gray, C.J., J.S. Narang, and S.A. Barker, Immobilization of Lipase from Candida cylindraceae and Its Use in the Synthesis of Menthol Esters by Transesterification, Enzyme Microb. Technol. 12:800–807 (1990).
Macrae, A.R., J.A. Bosley, and A.D. Peilow, Process for the Preparation of Fatty Acid Esters, European Patent Application EP 322,213 (1989).
Stark M.-B., and K. Holmberg, Covalent Immobilisation of Lipase in Organic Solvents, Biotechnol. Bioeng. 34:942–950 (1989).
Shaw, J.-F., R.-C. Chang, F.F. Wang, and H.J. Wang, Lipolytic Activities of a Lipase Immobilized on Six Selected Supporting Materials, Ibid.:132–137 (1990).
Telefoncu, A., E. Dinckaya, and K.D. Vorlop, Preparation and Characterization of Pancreatic Lipase Immobilized on Eudragit Matrix, Appl. Biochem. Biotechnol. 26:311–317 (1990).
Bosley, J.A., and J.C. Clayton, Blueprint for a Lipase Support: Use of Hydrophobic Controlled-Pore Glasses as Model Systems, Biotech. Bioeng. 43:934–938 (1994).
Montero, S., A. Blanco, M.D. Virto, L.C. Landeta, I. Agud, R. Solozabel, J.M. Lascaray, M. De Renobles, M.J. Llama, and J.L. Serra, Immobilization of Candida rugosa Lipase and Some Properties of the Immobilized Enzyme, Enzyme Microb. Technol. 15:239–247 (1993).
Huang, F.-C., and Y.-H. Ju, Improved Activity of Lipase by Vacuum Drying on Hydrophobic Microporous Support, Biotechnol. Tech. 8:827–830 (1994).
Bailie, P.M., S.E. McNerlan, E. Robinson, and W.R. Murphy, The Immobilization of Lipases for the Hydrolysis of Fats and Oils, IChemE. 73:71–76 (1995).
Analytical Method AF 95.1/3-GB, Novo-Nordisk, Copenhagen, Denmark. (1983).
Bosley J.A., and A.D. Peilow, Supported Enzyme, European Patent Application EP 424,130 (1991).
Author information
Authors and Affiliations
About this article
Cite this article
Bosley, J.A., Peilow, A.D. Immobilization of lipases on porous polypropylene: Reduction in esterification efficiency at low loading. J Amer Oil Chem Soc 74, 107–111 (1997). https://doi.org/10.1007/s11746-997-0153-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-997-0153-6