Abstract
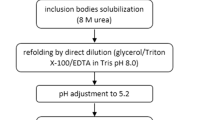
The fed-batch process using glucose as the sole source of carbon and energy with exponential feeding rate was carried out for high cell density cultivation of recombinant Escherichia coli BL21 (DE3) expressing human granulocyte-colony stimulating factor (hG-CSF). IPTG was used to induce the expression of hG-CSF at 48 g dry cell wt l−1 during high cell density culture of recombinant E. coli BL21 (DE3) [pET23a-g-csf]. The final cell density, specific yield and overall productivity of hG-CSF were obtained as ~64 g dry cell wt l−1, 223 mg hG-CSF g−1 dry cell wt and 775 mg hG-CSF l−1 h−1, respectively. The resulting purification process used cell lysis, inclusion body (IB) preparation, refolding, DEAE and Butyl-Sepharose. Effects of different process conditions such as cell lysis and washing of IB were evaluated. The results reveal that the cells lyzed at 1,200 bar, 99.9% and Triton removed about 64% of the LPS but sarcosyl had no effect on removal of nucleic acids and LPS. Further analysis show that DEAE column removes DNA about 84%. Cupper concentration was identified as parameter that could have a significant impact on aggregation, as an unacceptable pharmaceutical form that decrease process yields. The purity of purified hG-CSF was more than 99%. Also the comparison of activity between purified hG-CSF and commercial form do not show valuable decrease in activity in purified form.






Similar content being viewed by others
References
Riesenberg D, Guthke R (1999) High-cell-density cultivation of Escherichia coli. Curr Opin Biotechnol 2:380–384. doi:10.1016/S0958-1669(05)80142-9
Lee SY (1996) High cell-density culture of Escherichia coli. Trends Biotechnol 14:98–105. doi:10.1016/0167-7799(96)80930-9
Lim HK, Jung KH, Park DH, Chung SI (2000) Production characteristics of interferon-α using an L-arabinose promoter system in a high-cell-density culture. Appl Microbiol Biotechnol 53:201–208. doi:10.1007/s002530050009
Basu S, Dunn A, Ward A (2002) G-CSF: function and modes of action. Int J Mol Med 10:3–10
Hill CP, Osslund TD, Eisenberg D (1993) The structure of granulocyte-colony-stimulating factor and its relationship to other growth factors. Proc Natl Acad Sci USA 90:5167–5171. doi:10.1073/pnas.90.11.5167
Welte BK, Gabrilove J, Bronchud MH, Platzer E, Morstyn G (1996) Filgrastim (r-metHuG-CSF): the first 10 years. Blood 88(6):1907–1929
Kuga T, Komatsu Y, Yamasaki M, Sakine S, Miyaji H, Nishi T et al (1989) Mutagenesis of human granulocyte colony stimulating factor. Biochem Biophys Res Commun 159(1):103–111. doi:10.1016/0006-291X(89)92410-8
Wingfield P, Benedict R, Turcatti G, Allet B, Mermod JJ, DeLamarter J et al (1988) Characterization of recombinant-derived granulocyte-colony stimulating factor (G-CSF). Biochem J 256:213–218
Lu HS, Clogston CL, Narhi LO, Merewether LA, Pearl WR, Boone TC (1992) Folding and oxidation of recombinant human granulocyte colony stimulating factor produced in Escherichia coli. J Biol Chem 267(13):8770–8777
Komatsu Y, Matsumoto T, Kuga T, Nishi T, Sakine S, Saito A (1987) Cloning of granulocyte colony stimulating factor cDNA and its expression in Escherichia coli. Jpn J Cancer Res 78:1179–1181
Devlin PE, Drummond RJ, Toy P, Mark DF, Watt KW, Devlin JJ (1988) Alteration of amino-terminal codons of human granulocyte colony stimulating factor increases expression levels and allow efficient processing by methionine aminopeptidase in Escherichia coli. Gene 65:13–22. doi:10.1016/0378-1119(88)90412-X
Soo-Hyung K, Kyu-Heum N, Jang-Hyeon P, Choong-II P, Se-Yong L, Young Ik L (1995) High level expression and simple purification of recombinant human granulocyte colony stimulating factor in E. coli. Biotechnol Lett 17:687–692. doi:10.1007/BF00130351
Bishop B, Koay DC, Sartorelli AC, Regan L (2001) Reengineering granulocyte-colony stimulating factor (G-CSF) for enhanced stability. J Biol Chem 276:33465–33470. doi:10.1074/jbc.M104494200
Fallah MJ, Akbari B, Saeedinia AR, Karimi M, Zeinoddini M, Soleimani M (2003) Over expression of recombinant human granulocyte colony stimulating factor in E. coli. IJMS 28:131–134
Khalilzadeh R, Shojaosadati SA, Maghsoudi N, Mohammadian-Mosaabadi J, Mohammadi MR, Bahrami A et al (2004) Process development for production of recombinant human interferon-γ expressed in Escherichia coli. J Ind Microbiol Biotechnol 31:63–69. doi:10.1007/s10295-004-0117-x
Khalilzadeh R, Shojaosadati SA, Bahrami A, Maghsoudi N (2003) Over-expression of recombinant human interferon-gamma in high cell density fermentation of recombinant Escherichia coli. Biotechnol Lett 25:1989–1992. doi:10.1023/B:BILE.0000004390.98648.25
Mulkerrin MG, Wetzel R (1989) pH dependence of the reversible and irreversible thermal denaturation of gamma interferon. Biochemistry 28:6556–6561. doi:10.1021/bi00442a005
Seeger A, Schneppe B, McCarthy JEG, Deckwer W-D, Rinas U (1995) Comparison of temperature- and isopropyl-β-d-thiogalacto-pyranoside-induced synthesis of basic fibroblast growth factor in high-cell-density cultures of recombinant Escherichia coli. Enzyme Microb Technol 17:947–953. doi:10.1016/0141-0229(94)00123-9
Panda AK, Khan RH, Appa Rao KBC, Totey SM (1999) Kinetics of inclusion body production in batch and high cell density fed-batch culture of Escherichia coli expressing ovine growth hormone. J Biotechnol 75:161–172. doi:10.1016/S0168-1656(99)00157-1
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle protein dyebinding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Oshima Y, Tojo A, Niho Y, Asano S (2000) Biological activity of human granulocyte colony stimulating factor with a modified C-terminus. Biochem Biophys Res Commun 267:924–927. doi:10.1006/bbrc.1999.2062
Kleman GL, Strohl WR (1994) Development in high cell density and high productivity microbial fermentation. Curr Opin Biotechnol 5:180–186. doi:10.1016/S0958-1669(05)80033-3
Shiloach J, Kaufman J, Guillard AS, Fass R (1996) Effect of glucose supply strategy on acetate accumulation, growth, and recombinant protein production by Escherichia coli JM109. Biotechnol Bioeng 49:421–428. doi :10.1002/(SICI)1097-0290(19960220)49:4<421::AID-BIT9>3.0.CO;2-R
Saxena P, Wetlaufer DB (1970) Formation of three-dimensional structure in proteins. I. Rapid nonenzymatic activation of reduced lysozyme. Biochemistry 9:5015–5023. doi:10.1021/bi00827a028
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalilzadeh, R., Mohammadian-Mosaabadi, J., Bahrami, A. et al. Process development for production of human granulocyte-colony stimulating factor by high cell density cultivation of recombinant Escherichia coli . J Ind Microbiol Biotechnol 35, 1643–1650 (2008). https://doi.org/10.1007/s10295-008-0408-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0408-8