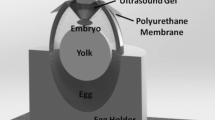
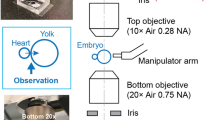
Abstract
Abnormal blood flow mechanics can result in pathological heart malformation, underlining the importance of understanding embryonic cardiac fluid mechanics. In the current study, we performed image-based computational fluid dynamics simulation of the zebrafish embryonic heart ventricles and characterized flow mechanics, organ dynamics, and energy dynamics in detail. 4D scans of 5 days post-fertilization embryonic hearts with GFP-labelled myocardium were acquired using line-scan focal modulation microscopy. This revealed that the zebrafish hearts exhibited a wave-like contractile/relaxation motion from the inlet to the outlet during both systole and diastole, which we showed to be an energy efficient configuration. No impedance pumping effects of pressure and velocity waves were observed. Due to its tube-like configuration, inflow velocities were higher near the inlet and smaller at the outlet and vice versa for outflow velocities. This resulted in an interesting spatial wall shear stress (WSS) pattern where WSS waveforms near the inlet and those near the outlet were out of phase. There was large spatial variability in WSS magnitudes. Peak WSS was in the range of 47.5–130 dyne/cm2 at the inflow and outflow tracts, but were much smaller, in the range of 4–11 dyne/cm2, in the mid-ventricular segment. Due to very low Reynolds number and the highly viscous environment, intraventricular pressure gradients were high, suggesting substantial energy losses of flow through the heart.







Similar content being viewed by others
References
Antiga L, Piccinelli M, Botti L, Ene-Iordache B, Remuzzi A, Steinman DA (2008) An image-based modeling framework for patient-specific computational hemodynamics. Med Biol Eng Comput 46:1097–1112
Avrahami I, Gharib M (2008) Computational studies of resonance wave pumping in compliant tubes. J Fluid Mech 608:139–160
Bark DL Jr, Johnson B, Garrity D, Dasi LP (2017) Valveless pumping mechanics of the embryonic heart during cardiac looping: pressure and flow through micro-PIV. J Biomech 50:50–55. https://doi.org/10.1016/j.jbiomech.2016.11.036
Bartman T et al (2004) Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol 2:e129
Chong SP, Pant S, Chen N (2011) Line-scan focal modulation microscopy for rapid imaging of thick biological specimens. In: Asia communications and photonics conference and exhibition. Optical Society of America, p 83111C
Feintuch A et al (2007) Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am J Physiol Heart Circ Physiol 292:H884–H892. https://doi.org/10.1152/ajpheart.00796.2006
Forouhar AS et al (2006) The embryonic vertebrate heart tube is a dynamic suction pump. Science 312:751–753. https://doi.org/10.1126/science.1123775
Geva T, Powell AJ, Crawford EC, Chung T, Colan SD (1998) Evaluation of regional differences in right ventricular systolic function by acoustic quantification echocardiography and cine magnetic resonance imaging. Circulation 98:339–345
Groenendijk BC, Hierck BP, Gittenberger-de Groot AC, Poelmann RE (2004) Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev Dyn 230:57–68
Haack T, Abdelilah-Seyfried S (2016) The force within: endocardial development, mechanotransduction and signalling during cardiac morphogenesis. Development (Cambridge, England) 143:373–386. https://doi.org/10.1242/dev.131425
Ho S, Tan GXY, Foo TJ, Phan-Thien N, Yap CH (2017) Organ dynamics and fluid dynamics of the HH25 chick embryonic cardiac ventricle as revealed by a novel 4D high-frequency ultrasound imaging technique and computational flow simulations. Ann Biomed Eng 45:2309–2323
Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M (2003) Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421:172–177. https://doi.org/10.1038/nature01282
Hu N, Sedmera D, Yost HJ, Clark EB (2000) Structure and function of the developing zebrafish heart. Anat Rec 260:148–157
Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH (2004) Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305:1007–1009. https://doi.org/10.1126/science.1100035
Jacob E, Drexel M, Schwerte T, Pelster B (2002) Influence of hypoxia and of hypoxemia on the development of cardiac activity in zebrafish larvae. Am J Physiol Regul Integr Comput Physiol 283:R911–917. https://doi.org/10.1152/ajpregu.00673.2001
Jamison RA, Fouras A, Bryson-Richardson RJ (2012) Cardiac-phase filtering in intracardiac particle image velocimetry. J Biomed Opt 17:036007. https://doi.org/10.1117/1.JBO.17.3.036007
Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EH (2008) Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322:1065–1069
Lee J et al (2013) Moving domain computational fluid dynamics to interface with an embryonic model of cardiac morphogenesis. PLoS ONE 8:e72924. https://doi.org/10.1371/journal.pone.0072924
Lee J et al (2017) A rapid capillary-pressure driven micro-channel to demonstrate newtonian fluid behavior of zebrafish blood at high shear rate. Sci Rep 7:1980. https://doi.org/10.1038/s41598-017-02253-7
Marstal K, Berendsen F, Staring M, Klein S (2016) SimpleElastix: a user-friendly, multi-lingual library for medical image registration. In: Proceedings of the IEEE conference on computer vision and pattern recognition workshops, pp 134–142
Mickoleit M, Schmid B, Weber M, Fahrbach FO, Hombach S, Reischauer S, Huisken J (2014) High-resolution reconstruction of the beating zebrafish heart. Nat Methods 11:919–922. https://doi.org/10.1038/nmeth.3037
Miller LA (2011) Fluid dynamics of ventricular filling in the embryonic heart. Cell Biochem Biophys 61:33–45. https://doi.org/10.1007/s12013-011-9157-9
Mosimann C, Puller AC, Lawson KL, Tschopp P, Amsterdam A, Zon LI (2013) Site-directed zebrafish transgenesis into single landing sites with the phiC31 integrase system. Dev Dyn 242:949–963. https://doi.org/10.1002/dvdy.23989
Oslash RB, Weber R (1995) Effects of oxygenation and the stress hormones adrenaline and cortisol on the viscosity of blood from the trout Oncorhynchus mykiss. J Exp Biol 198:953–959
Pant S, Duan Y, Xiong F, Chen N (2017a) Augmented line-scan focal modulation microscopy for multi-dimensional imaging of zebrafish heart in vivo. Biomed Opt Express 8:5698–5707
Pant S, Li C, Gong Z, Chen N (2017b) Line-scan focal modulation microscopy. J Biomed Opt 22:50502. https://doi.org/10.1117/1.JBO.22.5.050502
Pantokratoras A (2016) Steady laminar flow in a 90 bend. Adv Mech Eng 8:1687814016669472
Peterson LM, Jenkins MW, Gu S, Barwick L, Watanabe M, Rollins AM (2012) 4D shear stress maps of the developing heart using Doppler optical coherence tomography. Biomed Opt Express 3:3022–3032. https://doi.org/10.1364/boe.3.003022
Raines R, LeWinter M, Covell J (1976) Regional shortening patterns in canine right ventricle. Am J Physiol Leg Content 231:1395–1400
Schenkel T, Malve M, Reik M, Markl M, Jung B, Oertel H (2009) MRI-based CFD analysis of flow in a human left ventricle: methodology and application to a healthy heart. Ann Biomed Eng 37:503–515. https://doi.org/10.1007/s10439-008-9627-4
Scherz PJ, Huisken J, Sahai-Hernandez P, Stainier DY (2008) High-speed imaging of developing heart valves reveals interplay of morphogenesis and function. Development (Cambridge, England) 135:1179–1187. https://doi.org/10.1242/dev.010694
Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB (1999) Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec 254:238–252
Staudt DW, Liu J, Thorn KS, Stuurman N, Liebling M, Stainier DY (2014) High-resolution imaging of cardiomyocyte behavior reveals two distinct steps in ventricular trabeculation. Development (Cambridge, England) 141:585–593. https://doi.org/10.1242/dev.098632
Steed E, Boselli F, Vermot J (2016a) Hemodynamics driven cardiac valve morphogenesis. Biochim Biophys Acta 1863:1760–1766. https://doi.org/10.1016/j.bbamcr.2015.11.014
Steed E, Faggianelli N, Roth S, Ramspacher C, Concordet J-P, Vermot J (2016b) klf2a couples mechanotransduction and zebrafish valve morphogenesis through fibronectin synthesis. Nat Commun 7:11646
Tobita K, Keller BB (2000) Right and left ventricular wall deformation patterns in normal and left heart hypoplasia chick embryos. Am J Physiol Heart Circ Physiol 279:H959–969
Topper JN, Gimbrone MA (1999) Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today 5:40–46. https://doi.org/10.1016/S1357-4310(98)01372-0
Tu S, Chi NC (2012) Zebrafish models in cardiac development and congenital heart birth defects. Differentiation 84:4–16. https://doi.org/10.1016/j.diff.2012.05.005
Vedula V, Lee J, Xu H, Kuo C-CJ, Hsiai TK, Marsden AL (2017) A method to quantify mechanobiologic forces during zebrafish cardiac development using 4-D light sheet imaging and computational modeling. PLoS Comput Biol 13:e1005828
Wiputra H et al (2016) Fluid mechanics of human fetal right ventricles from image-based computational fluid dynamics using 4D clinical ultrasound scans. Am J Physiol Heart Circ Physiol 311:H1498–H1508. https://doi.org/10.1152/ajpheart.00400.2016
Yotti R et al (2005) Doppler-derived ejection intraventricular pressure gradients provide a reliable assessment of left ventricular systolic chamber function. Circulation 112:1771–1779. https://doi.org/10.1161/CIRCULATIONAHA.104.485128
Acknowledgement
This study was supported by the National University of Singapore 2015 Young Investigator Award (PI:Yap). All authors have no conflict of interest to declare. We thank Christian Mosimann and Suresh Jesuthasan for providing the transgenic zebrafish line.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Foo, Y.Y., Pant, S., Tay, H. et al. 4D modelling of fluid mechanics in the zebrafish embryonic heart. Biomech Model Mechanobiol 19, 221–232 (2020). https://doi.org/10.1007/s10237-019-01205-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-019-01205-6