Abstract
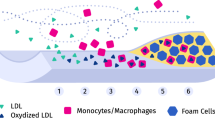
This work is devoted to the development of a mathematical model of the early stages of atherosclerosis incorporating processes of all time scales of the disease and to show their interactions. The cardiovascular mechanics is modeled by a fluid–structure interaction approach coupling a non-Newtonian fluid to a hyperelastic solid undergoing anisotropic growth and a change of its constitutive equation. Additionally, the transport of low-density lipoproteins and its penetration through the endothelium is considered by a coupled set of advection–diffusion-reaction equations. Thereby, the permeability of the endothelium is wall-shear stress modulated resulting in a locally varying accumulation of foam cells triggering a novel growth and remodeling formulation. The model is calibrated and applied to an murine-specific case study, and a qualitative validation of the computational results is performed. The model is utilized to further investigate the influence of the pulsatile blood flow and the compliance of the artery wall to the atherosclerotic process. The computational results imply that the pulsatile blood flow is crucial, whereas the compliance of the aorta has only a minor influence on atherosclerosis. Further, it is shown that the novel model is capable to produce a narrowing of the vessel lumen inducing an adaption of the endothelial permeability pattern.














Similar content being viewed by others
Notes
Wall-shear stresses is the established name even though wall-shear tractions would be more accurate
References
Ambrosi D, Mollica F (2002) On the mechanics of a growing tumor. Int J Eng Sci 40(12):1297–1316
Arora D (2005) Computational hemodynamics: Hemolysis and viscoelasticity. Ph.D. thesis, Citeseer
Asakura T, Karino T (1990) Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res 66(4):1045–1066
Aslanidou L, Trachet B, Reymond P, Fraga-Silva RA, Segers P, Stergiopulos N (2016) A 1D model of the arterial circulation in mice. ALTEX 33(1):13
Balzani D, Schmidt T (2015) Comparative analysis of damage functions for soft tissues: properties at damage initialization. Math Mech Solids 20(4):480–492
Barrenechea GR, Valentin F (2002) An unusual stabilized finite element method for a generalized stokes problem. Numer Math 92(4):653–677
Bazilevs Y, Calo VM, Tezduyar TE, Hughes TJ (2007) YZ\(\beta \) discontinuity capturing for advection-dominated processes with application to arterial drug delivery. Int J Numer Methods Fluids 54:593–608
Bazilevs Y, Gohean J, Hughes T, Moser R, Zhang Y (2009) Patient-specific isogeometric fluid-structure interaction analysis of thoracic aortic blood flow due to implantation of the Jarvik 2000 left ventricular assist device. Comput Methods Appl Mech Eng 198(45):3534–3550
Bird RB, Armstrong R, Hassager O (1987) Dynamics of polymeric liquids, vol 1. Fluid mechanics. Wiley, New York
Brown AJ, Teng Z, Evans PC, Gillard JH, Samady H, Bennett MR (2016) Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol 13:210–220
Calvez V, Houot JG, Meunier N, Raoult A, Rusnakova G (2010) Mathematical and numerical modeling of early atherosclerotic lesions. ESAIM Proc Surv 30:14. https://doi.org/10.1051/proc/2010002
Carew TE, VAISHNAV RN, PATEL DJ (1968) Compressibility of the arterial wall. Circ Res 23(1):61–68
Chalmers AD, Cohen A, Bursill CA, Myerscough MR (2015) Bifurcation and dynamics in a mathematical model of early atherosclerosis. J Math Biol 71(6–7):1451–1480
Chen J, Lu XY (2006) Numerical investigation of the non-newtonian pulsatile blood flow in a bifurcation model with a non-planar branch. J Biomech 39(5):818–832
Cheng C, Helderman F, Tempel D, Segers D, Hierck B, Poelmann R, van Tol A, Duncker DJ, Robbers-Visser D, Ursem NT et al (2007) Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis 195(2):225–235
Cho YI, Kensey KR (1991) Effects of the non-newtonian viscosity of blood on flows in a diseased arterial vessel. Part 1: steady flows. Biorheology 28:241–62
Cilla M, Peña E, Martínez MA (2014) Mathematical modelling of atheroma plaque formation and development in coronary arteries. J R Soc Interface 11(90):20130,866
Codina R (2002) Stabilized finite element approximation of transient incompressible flows using orthogonal subscales. Comput Methods Appl Mech Eng 191(39):4295–4321
Crosetto P, Reymond P, Deparis S, Kontaxakis D, Stergiopulos N, Quarteroni A (2011) Fluid–structure interaction simulation of aortic blood flow. Comput Fluids 43(1):46–57
De Souza Neto E, Perić D, Dutko M, Owen D (1996) Design of simple low order finite elements for large strain analysis of nearly incompressible solids. Int J Solids Struct 33(20):3277–3296
De Wilde D, Trachet B, De Meyer GR, Segers P (2015a) Shear stress metrics and their relation to atherosclerosis: an in vivo follow-up study in atherosclerotic mice. Ann Biomed Eng 44:2327–2388
De Wilde D, Trachet B, Debusschere N, Iannaccone F, Swillens A, Degroote J, Vierendeels J, De Meyer GR, Segers P (2015b) Assessment of shear stress related parameters in the carotid bifurcation using mouse-specific FSI simulations. J Biomech 49:2135–2142
De Wilde D, Trachet B, De Meyer G, Segers P (2016) The influence of anesthesia and fluid–structure interaction on simulated shear stress patterns in the carotid bifurcation of mice. J Biomech 49(13):2741–2747
Dobrin PB, Rovick AA (1969) Influence of vascular smooth muscle on contractile mechanics and elasticity of arteries. Am J Physiol Leg Content 217(6):1644–1651
Doll S, Schweizerhof K (2000) On the development of volumetric strain energy functions. J Appl Mech 67(1):17–21
Doll S, Schweizerhof K, Hauptmann R, Freischläger C (2000) On volumetric locking of low-order solid and solid-shell elements for finite elastoviscoplastic deformations and selective reduced integration. Eng Comput 17(7):874–902
Donea J, Huerta A (2003) Finite element methods for flow problems. Wiley, Hoboken
Donea J, Giuliani S, Halleux J (1982) An arbitrary lagrangian-eulerian finite element method for transient dynamic fluid–structure interactions. Comput Methods Appl Mech Eng 33(1):689–723
Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP et al (2004) Atherosclerotic vascular disease conference writing group iii: pathophysiology. Circulation 109(21):2617–2625
Feintuch A, Ruengsakulrach P, Lin A, Zhang J, Zhou YQ, Bishop J, Davidson L, Courtman D, Foster FS, Steinman DA et al (2007) Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am J Physiol Heart Circ Physiol 292(2):H884–H892
Ferruzzi J, Bersi M, Humphrey J (2013) Biomechanical phenotyping of central arteries in health and disease: advantages of and methods for murine models. Ann Biomed Eng 41(7):1311–1330
Figueroa CA, Baek S, Taylor CA, Humphrey JD (2009) A computational framework for fluid–solid-growth modeling in cardiovascular simulations. Comput Methods Appl Mech Eng 198(45–46):3583–3602. https://doi.org/10.1016/j.cma.2008.09.013
Filipovic N, Rosic M, Tanaskovic I, Parodi O, Fotiadis D (2011) Computer simulation and experimental analysis of LDL transport in the arteries. In: Engineering in medicine and biology society, EMBC, 2011 Annual International Conference of the IEEE. IEEE, pp 195–198
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Friedman A, Hao W (2015) A mathematical model of atherosclerosis with reverse cholesterol transport and associated risk factors. Bull Math Biol 77(5):758–781
Gee M, Förster C, Wall W (2010) A computational strategy for prestressing patient-specific biomechanical problems under finite deformation. Int J Numer Methods Biomed Eng 26(1):52–72
Gee MW, Küttler U, Wall WA (2011) Truly monolithic algebraic multigrid for fluid–structure interaction. Int J Numer Methods Eng 85(8):987–1016
Gijsen F, Allanic E, Van de Vosse F, Janssen J (1999) The influence of the non-newtonian properties of blood on the flow in large arteries: unsteady flow in a 90 curved tube. J Biomech 32(7):705–713
Goriely A, Amar MB (2007) On the definition and modeling of incremental, cumulative, and continuous growth laws in morphoelasticity. Biomech Model Mechanobiol 6(5):289–296
Gravemeier V, Comerford A, Yoshihara L, Ismail M, Wall WA (2012) A novel formulation for Neumann inflow boundary conditions in biomechanics. Int J Numer Methods Biomed Eng 28(5):560–573
Haskett D, Johnson G, Zhou A, Utzinger U, Geest JV (2010) Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomech Model Mechanobiol 9(6):725–736
Heiland VM, Forsell C, Roy J, Hedin U, Gasser TC (2013) Identification of carotid plaque tissue properties using an experimental-numerical approach. J Mech Behav Biomed Mater 27:226–238
Herrmann RA, Malinauskas RA, Truskey GA (1994) Characterization of sites with elevated ldl permeability at intercostal, celiac, and iliac branches of the normal rabbit aorta. Arterioscler Thromb Vasc Biol 14(2):313–323
Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH (2004) Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol 286(5):H1916–H1922
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast Phys Sci Solids 61(1–3):1–48
Holzapfel GA, Mulvihill JJ, Cunnane EM, Walsh MT (2014) Computational approaches for analyzing the mechanics of atherosclerotic plaques: a review. J Biomech 47(4):859–869
Hossain SS, Hossainy SF, Bazilevs Y, Calo VM, Hughes TJ (2012) Mathematical modeling of coupled drug and drug-encapsulated nanoparticle transport in patient-specific coronary artery walls. Comput Mech 49(2):213–242
Humphrey J (2002) Cardiovascular solid mechanics: cells, tissues, and organs. Springer, Berlin
Huo Y, Guo X, Kassab GS (2008) The flow field along the entire length of mouse aorta and primary branches. Ann Biomed Eng 36(5):685–699
Ismail M, Wall WA, Gee MW (2013) Adjoint-based inverse analysis of windkessel parameters for patient-specific vascular models. J Comput Phys 244:113–130
Ismail M, Gravemeier V, Comerford A, Wall W (2014) A stable approach for coupling multidimensional cardiovascular and pulmonary networks based on a novel pressure-flow rate or pressure-only Neumann boundary condition formulation. Int J Numer Methods Biomed Eng 30(4):447–469
Johnston BM, Johnston PR, Corney S, Kilpatrick D (2004) Non-Newtonian blood flow in human right coronary arteries: steady state simulations. J Biomech 37(5):709–720
Južnič G, Klensch H (1964) Vergleichend-physiologische Untersuchungen über das Verhalten der Indices für Energieaufwand und Leistung des Herzens. Pflüger’s Archiv für die gesamte Physiologie des Menschen und der Tiere 280(1):38–45
Karimi R, Zhu T, Bouma BE, Mofrad MRK (2008) Estimation of nonlinear mechanical properties of vascular tissues via elastography. Cardiovasc Eng 8(4):191–202
Karner G, Perktold K (2000) Effect of endothelial injury and increased blood pressure on albumin accumulation in the arterial wall: a numerical study. J Biomech 33(6):709–715
Kedem O, Katchalsky A (1958) Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta 27:229–246
Klisch SM, Van Dyke TJ, Hoger A (2001) A theory of volumetric growth for compressible elastic biological materials. Math Mech Solids 6(6):551–575
Klöppel T, Popp A, Küttler U, Wall WA (2011) Fluid–structure interaction for non-conforming interfaces based on a dual mortar formulation. Comput Methods Appl Mech Eng 200(45):3111–3126
Koshiba N, Ando J, Chen X, Hisada T (2007) Multiphysics simulation of blood flow and LDL transport in a porohyperelastic arterial wall model. J Biomech Eng 129(3):374–385
Ku DN, Giddens DP, Zarins CK, Glagov S (1985) Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscler Thromb Vasc Biol 5(3):293–302
Kuhl E, Maas R, Himpel G, Menzel A (2007) Computational modeling of arterial wall growth. Biomech Model Mechanobiol 6(5):321–331
Küttler U, Gee M, Förster C, Comerford A, Wall W (2010) Coupling strategies for biomedical fluid–structure interaction problems. Int J Numer Methods Biomed Eng 26(3–4):305–321
Kuzmin D (2010) A guide to numerical methods for transport equations. University Erlangen-Nuremberg, Erlangen
Lee SW, Antiga L, Spence JD, Steinman DA (2008) Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke 39(8):2341–2347
Liu M, Tang H, Nicholson JK, Lindon JC (2002) Use of 1H NMR-determined diffusion coefficients to characterize lipoprotein fractions in human blood plasma. Magn Reson Chem 40(13):S83–S88
Liu Y, Dang C, Garcia M, Gregersen H, Kassab GS (2007) Surrounding tissues affect the passive mechanics of the vessel wall: theory and experiment. Am J Physiol Heart Circ Physiol 293(6):H3290–H3300
Liu X, Fan Y, Deng X (2010) Effect of spiral flow on the transport of oxygen in the aorta: a numerical study. Ann Biomed Eng 38(3):917–926
Liu X, Fan Y, Deng X, Zhan F (2011) Effect of non-Newtonian and pulsatile blood flow on mass transport in the human aorta. J Biomech 44(6):1123–1131
Loree HM, Tobias BJ, Gibson LJ, Kamm RD, Small DM, Lee RT (1994) Mechanical properties of model atherosclerotic lesion lipid pools. Arterioscler Thromb Vasc Biol 14(2):230–234
Lutgens E, Lievens D, Beckers L, Wijnands E, Soehnlein O, Zernecke A, Seijkens T, Engel D, Cleutjens J, Keller AM et al (2010) Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med 207(2):391–404
Mayr M, Klöppel T, Wall WA, Gee MW (2015) A temporal consistent monolithic approach to fluid-structure interaction enabling single field predictors. SIAM J Sci Comput 37(1):B30–B59
Moireau P, Xiao N, Astorino M, Figueroa CA, Chapelle D, Taylor CA, Gerbeau JF (2012) External tissue support and fluid–structure simulation in blood flows. Biomech Model Mechanobiol 11(1–2):1–18
Nadkarni SK, Bouma BE, Helg T, Chan R, Halpern E, Chau A, Minsky MS, Motz JT, Houser SL, Tearney GJ (2005) Characterization of atherosclerotic plaques by laser speckle imaging. Circulation 112(6):885–892
Ogden R (1972) Large deformation isotropic elasticity-on the correlation of theory and experiment for incompressible rubberlike solids. In: Proceedings of the royal society of London A: mathematical, physical and engineering sciences, The Royal Society, vol. 326, pp 565–584
Ogden R (1978) Nearly isochoric elastic deformations: application to rubberlike solids. J Mech Phys Solids 26(1):37–57
Olgac U, Poulikakos D, Saur SC, Alkadhi H, Kurtcuoglu V (2009) Patient-specific three-dimensional simulation of LDL accumulation in a human left coronary artery in its healthy and atherosclerotic states. Am J Physiol Heart Circ Physiol 296(6):H1969–H1982
Olshanskii M, Lube G, Heister T, Löwe J (2009) Grad-div stabilization and subgrid pressure models for the incompressible Navier–Stokes equations. Comput Methods Appl Mech Eng 198(49):3975–3988
Olufsen MS, Peskin CS, Kim WY, Pedersen EM, Nadim A, Larsen J (2000) Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann Biomed Eng 28(11):1281–1299
Ougrinovskaia A, Thompson RS, Myerscough MR (2010) An ode model of early stages of atherosclerosis: mechanisms of the inflammatory response. Bull Math Biol 72(6):1534–61. https://doi.org/10.1007/s11538-010-9509-4
Parton A, McGilligan V, O’Kane M, Baldrick FR, Watterson S (2016) Computational modelling of atherosclerosis. Brief Bioinform 17(4): 562–575. https://doi.org/10.1093/bib/bbv081
Peiffer V, Sherwin SJ, Weinberg PD (2013) Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? a systematic review. Cardiovasc Res 99(2):242–250. https://doi.org/10.1093/cvr/cvt044
Prosi M, Zunino P, Perktold K, Quarteroni A (2005) Mathematical and numerical models for transfer of low-density lipoproteins through the arterial walls: a new methodology for the model set up with applications to the study of disturbed lumenal flow. J Biomech 38(4):903–917
Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LCM, Wofovitz E (2003) Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol 81(3):177–199
Roccabianca S, Figueroa C, Tellides G, Humphrey J (2014) Quantification of regional differences in aortic stiffness in the aging human. J Mech Behav Biomed Mater 29:618–634
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340(2):115–126
Saad Y, Schultz MH (1986) GMRES: a generalized minimal residual algorithm for solving nonsymmetric linear systems. SIAM J Sci Stat Comput 7(3):856–869
Sansour C (2008) On the physical assumptions underlying the volumetric-isochoric split and the case of anisotropy. Eur J Mech A Solids 27(1):28–39
Skalak R, Zargaryan S, Jain RK, Netti PA, Hoger A (1996) Compatibility and the genesis of residual stress by volumetric growth. J Math Biol 34(8):889–914
Smith N, Pullan A, Hunter PJ (2002) An anatomically based model of transient coronary blood flow in the heart. SIAM J Appl Math 62(3):990–1018
Soulis JV, Lampri OP, Fytanidis DK, Giannoglou GD (2011) Relative residence time and oscillatory shear index of non-Newtonian flow models in aorta. In: 2011 10th international workshop on biomedical engineering, IEEE, pp 1–4
Stangeby DK, Ethier CR (2002) Computational analysis of coupled blood-wall arterial LDL transport. J Biomech Eng 124(1):1–8
Stocker R, Keaney JF (2004) Role of oxidative modifications in atherosclerosis. Physiol Rev 84(4):1381–1478
Sun N, Wood NB, Hughes AD, Thom SAM, Xu XY (2007) Influence of pulsatile flow on ldl transport in the arterial wall. Ann Biomed Eng 35(10):1782–90. https://doi.org/10.1007/s10439-007-9347-1
Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP (2007) Hemodynamic shear stresses in mouse aortas implications for atherogenesis. Arterioscler Thromb Vasc Biol 27(2):346–351
Thomas SR, Mikulecky DC (1978) Transcapillary solute exchange: a comparison of the kedem-katchalsky convection–diffusion equations with the rigorous nonlinear equations for this special case. Microvasc Res 15(2):207–220
Tomaso GD, Díaz-Zuccarini V, Pichardo-Almarza C (2011) A multiscale model of atherosclerotic plaque formation at its early stage. IEEE Trans Biomed Eng 58(12):3460–3463
Tompkins RG (1991) Quantitative analysis of blood vessel permeability of squirrel monkeys. Am J Physiol Heart Circ Physiol 260(4):H1194–H1204
Truskey GA, Roberts WL, Herrmann RA, Malinauskas RA (1992) Measurement of endothelial permeability to 125i-low density lipoproteins in rabbit arteries by use of en face preparations. Circ Res 71(4):883–897
Wada S, Koujiya M, Karino T (2002) Theoretical study of the effect of local flow disturbances on the concentration of low-density lipoproteins at the luminal surface of end-to-end anastomosed vessels. Med Biol Eng Compu 40(5):576–587
Wall W, Gee M (2010) Baci: a parallel multiphysics finite element environment. Technische Universität München, Institute for Computational Mechanics
Wang W, Lee Y, Lee CH (2013) Review: the physiological and computational approaches for atherosclerosis treatment. Int J Cardiol 167(5):1664–1676
Westerhof N, Lankhaar JW, Westerhof BE (2009) The arterial windkessel. Med Biol Eng Comput 47(2):131–141
Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG (2004) Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol 286(6):H2408–H2415
Wiesmann F, Szimtenings M, Frydrychowicz A, Illinger R, Hunecke A, Rommel E, Neubauer S, Haase A (2003) High-resolution mri with cardiac and respiratory gating allows for accurate in vivo atherosclerotic plaque visualization in the murine aortic arch. Magn Reson Med 50(1):69–74
Xiao N, Alastruey J, Alberto Figueroa C (2014) A systematic comparison between 1-D and 3-D hemodynamics in compliant arterial models. Int J Numer Methods Biomed Eng 30(2):204–31. https://doi.org/10.1002/cnm.2598
Yang N, Vafai K (2006) Modeling of low-density lipoprotein (LDL) transport in the artery—effects of hypertension. Int J Heat Mass Transf 49(5):850–867
Yang Y, Richter T, Jäger W, Neuss-Radu M (2016) An ALE approach to mechano-chemical processes in fluid-structure interactions. Int J Numer Methods Fluids 84:199–220
Yoshihara L, Coroneo M, Comerford A, Bauer G, Klöppel T, Wall W (2014) A combined fluid–structure interaction and multi-field scalar transport model for simulating mass transport in biomechanics. Int J Numer Methods Eng 100(4):277–299
Zareh M, Fradet G, Naser G, Mohammadi H (2015) Are two-dimensional images sufficient to assess the atherosclerotic plaque vulnerability: a viscoelastic and anisotropic finite element model. Cardiovasc Syst 3(1):3
Zunino P (2002) Mathematical and numerical modeling of mass transfer in the vascular system. Ph.D. thesis, Politecnico di Milano
Acknowledgements
The authors gratefully acknowledge the financial support given by the International Graduate School of Science and Engineering of the Technical University of Munich under the project BioMat01, A Multiscale Model of Atherosclerosis. This work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no conflicts of interest exist.
Rights and permissions
About this article
Cite this article
Thon, M.P., Hemmler, A., Glinzer, A. et al. A multiphysics approach for modeling early atherosclerosis. Biomech Model Mechanobiol 17, 617–644 (2018). https://doi.org/10.1007/s10237-017-0982-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-017-0982-7