Abstract
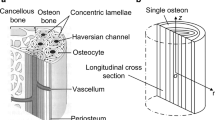
Trabecula, an anatomical unit of the cancellous bone, is a porous material that consists of a lamellar bone matrix and interstitial fluid in a lacuno-canalicular porosity. The flow of interstitial fluid caused by deformation of the bone matrix is believed to initiate a mechanical response in osteocytes for bone remodeling. In order to clarify the effect of the lamellar structure of the bone matrix—i.e., variations in material properties—on the fluid flow stimuli to osteocytes embedded in trabeculae, we investigated the mechanical behavior of an individual trabecula subjected to cyclic loading based on poroelasticity. We focused on variations in the trabecular permeability and developed an analytical solution containing both transient and steady-state responses for interstitial fluid pressure in a single trabecular model represented by a multilayered two-dimensional poroelastic slab. Based on the obtained solution, we calculated the pressure and seepage velocity of the interstitial fluid in lacuno-canalicular porosity, within the single trabecula, under various permeability distributions. Poroelastic analysis showed that a heterogeneous distribution of permeability produces remarkable variations in the fluid pressure and seepage velocity in the cross section of the individual trabecula, and suggests that fluid flow stimuli to osteocytes are mostly governed by the value of permeability in the neighborhood of the trabecular surfaces if there is no difference in the average permeability in a single trabecula.






Similar content being viewed by others
References
Beno T, Yoon YJ, Cowin SC, Fritton SP (2006) Estimation of bone permeability using accurate microstructural measurements. J Biomech 39:2378–2387
Biot MA (1941) General theory of three-dimensional consolidation. J Appl Phys 12:155–164
Biot MA (1955) Theory of elasticity and consolidation for a porous anisotropic solid. J Appl Phys 26:182–185
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238
Burger EH, Klein-Nulend J (1999) Mechanotransduction in bone-role of the lacuno-canalicular network. FASEB J 13:S101–S112
Coussy O (2004) Poromechanics. Wiley, West Sussex
Cowin SC (1999) Bone poroelasticity. J Biomech 32:217–238
Cowin SC, Moss-Salentijn L, Moss ML (1991) Candidates for the mechanosensory system in bone. J Biomech Eng 113:191–197
Detournay E, Cheng AH-D (1993) Fundamentals of poroelasticity. Comprehensive rock engineering: principles, practice and projects. In: Fairhurst C (ed) Analysis and design method, vol II. Pergamon Press, Oxford, pp 113–171 (Chapter 5)
Frost HM (1987) Bone ”mass” and the ”mechanostat”: a proposal. Anat Rec 219:1–9
Frost HM (2003) Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol 275:1081–1101
Gailani G, Benalla M, Mahamud R, Cowin SC, Cardoso L (2009) Experimental determination of the permeability in the lacunar-canalicular porosity of bone. J Biomech Eng 131:101007
Goldstein SA, Matthews LS, Kuhn JL, Hollister SJ (1991) Trabecular bone remodeling: an experimental model. J Biomech 24:135–150
Himeno-Ando A, Izumi Y, Yamaguchi A, Iimura T (2012) Structural differences in the osteocyte network between the calvaria and long bone revealed by three-dimensional fluorescence morphometry, possibly reflecting distinct mechano-adaptations and sensitivities. Biochem Biophys Res Commun 417:765–770
Kameo Y, Adachi T, Hojo M (2008) Transient response of fluid pressure in a poroelastic material under uniaxial cyclic loading. J Mech Phys Solids 56:1794–1805
Kameo Y, Adachi T, Hojo M (2009) Fluid pressure response in poroelastic materials subjected to cyclic loading. J Mech Phys Solids 57:1815–1827
Kameo Y, Adachi T, Hojo M (2010) Estimation of bone permeability considering the morphology of lacuno-canalicular porosity. J Mech Behav Biomed Mater 3:240–248
Kameo Y, Adachi T (2014) Interstitial fluid flow in canaliculi as a mechanical stimulus for cancellous bone remodeling: in silico validation. Biomech Model Mechanobiol 13:851–860
Manfredini P, Cocchetti G, Maier G, Redaelli A, Montevecchi FM (1999) Poroelastic finite element analysis of a bone specimen under cyclic loading. J Biomech 32:135–144
Martin RB, Burr DB, Sharley NA (1998) Skeletal tissue mechanics. Springer, New York
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:231–1234
Oyen ML (2008) Poroelastic nanoindentation responses of hydrated bone. J Mater Res 23:1307–1314
Parfitt AM (1994) Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 55:273–286
Pereira AF, Shefelbine SJ (2014) The influence of load repetition in bone mechanotransduction using poroelastic finite-element models: the impact of permeability. Biomech Model Mechanobiol 13:215–225
Price C, Zhou X, Li W, Wang L (2011) Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J Bone Miner Res 26:277–285
Qing H, Bonewald LF (2009) Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci 1:59–65
Rémond A, Naili S (2005) Transverse isotropic poroelastic osteon model under cyclic loading. Mech Res Commun 32:645–651
Rémond A, Naili S, Lemaire T (2008) Interstitial fluid flow in the osteon with spatial gradients of mechanical properties: a finite element study. Biomech Model Mechanobiol 7:487–495
Smit TH, Huyghe JM, Cowin SC (2002) Estimation of the poroelastic parameters of cortical bone. J Biomech 35:829–835
Sugawara Y, Kamioka H, Honjo T, Tezuka K, Takano-Yamamoto T (2005) Three-dimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone 36:877–883
Tatsumi S, Ishii K, Amizuka N, Li MQ, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 5:464–475
Turner VH, Rho J, Takano Y, Tsui TY, Pharr GM (1999) The elastic properties of trabecular and cortical bone tissues are similar: results from two microscopic measurement techniques. J Biomech 32:437–441
Wang HF (2000) Theory of Linear Poroelasticity with applications to geomechanics and hydrogeology. Princeton University Press, Princeton
Weinbaum S, Cowin SC, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27:339–360
Zeng Y, Cowin SC, Weinbaum S (1994) A fiber matrix model for fluid flow and streaming potentials in the canaliculi of an osteon. Ann Biomed Eng 22:280–292
Zhang D, Cowin SC (1994) Oscillatory bending of a poroelastic beam. J Mech Phys Solids 42:1575–1599
Zhang D, Weinbaum S, Cowin SC (1998) Estimates of the peak pressures in bone pore water. J Biomech Eng 120:697–703
Acknowledgments
This study was partially supported by a Grant-in-Aid for Young Scientists (B) (25820011) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kameo, Y., Ootao, Y. & Ishihara, M. Poroelastic analysis of interstitial fluid flow in a single lamellar trabecula subjected to cyclic loading. Biomech Model Mechanobiol 15, 361–370 (2016). https://doi.org/10.1007/s10237-015-0693-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-015-0693-x