Abstract
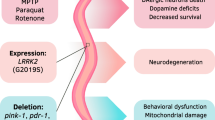
The nematode Caenorhabditis elegans has a very well-defined and genetically tractable nervous system which offers an effective model to explore basic mechanistic pathways that might be underpin complex human neurological diseases. Here, the role C. elegans is playing in understanding two neurodegenerative conditions, Parkinson’s and Alzheimer’s disease (AD), and a complex neurological condition, autism, is used as an exemplar of the utility of this model system. C. elegans is an imperfect model of Parkinson’s disease because it lacks orthologues of the human disease-related genes PARK1 and LRRK2 which are linked to the autosomal dominant form of this disease. Despite this fact, the nematode is a good model because it allows transgenic expression of these human genes and the study of the impact on dopaminergic neurons in several genetic backgrounds and environmental conditions. For AD, C. elegans has orthologues of the amyloid precursor protein and both human presenilins, PS1 and PS2. In addition, many of the neurotoxic properties linked with Aβ amyloid and tau peptides can be studied in the nematode. Autism spectrum disorder is a complex neurodevelopmental disorder characterised by impairments in human social interaction, difficulties in communication, and restrictive and repetitive behaviours. Establishing C. elegans as a model for this complex behavioural disorder is difficult; however, abnormalities in neuronal synaptic communication are implicated in the aetiology of the disorder. Numerous studies have associated autism with mutations in several genes involved in excitatory and inhibitory synapses in the mammalian brain, including neuroligin, neurexin and shank, for which there are C. elegans orthologues. Thus, several molecular pathways and behavioural phenotypes in C. elegans have been related to autism. In general, the nematode offers a series of advantages that combined with knowledge from other animal models and human research, provides a powerful complementary experimental approach for understanding the molecular mechanisms and underlying aetiology of complex neurological diseases.


Similar content being viewed by others
References
Abrahams BS, Geschwind DH (2008) Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 9(5):341–355
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136(1):317–324
Antoshechkin I, Sternberg PW (2007) The versatile worm: genetic and genomic resources for Caenorhabditis elegans research. Nat Rev 8:518–532
Asikainen S, Vartiainen S, Lakso M, Nass R, Wong G (2005) Selective sensitivity of Caenorhabditis elegans neurons to RNA interference. Neuroreport 16(18):1995–1999
Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E et al (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25(1):63–77
Baluchnejadmojarad T, Roghani M, Nadoushan MR, Bagheri M (2009) Neuroprotective effect of genistein in 6-hydroxydopamine hemi-parkinsonian rat model. Phytother Res 23(1):132–135
Bargmann CI (1998) Neurobiology of the Caenorhabditis elegans genome. Science 282(5396):2028–2033
Bartels T, Choi JG, Selkoe DJ (2011) Alpha-synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477(7362):107–110
Baudouin S, Scheiffele P (2010) SnapShot: neuroligin–neurexin complexes. Cell 141(5):908
Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U et al (2010) Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet 42(6):489–491
Bertram L, Lill CM, Tanzi RE (2010) The genetics of Alzheimer disease: back to the future. Neuron 68(2):270–281
Biswas S, Reinhard J, Oakeshott J, Russell R, Srinivasan MV, Claudianos C (2010) Sensory regulation of neuroligins and neurexin I in the honeybee brain. PLoS One 5(2):e9133
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E et al (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299(5604):256–259
Bourgeron T (2009) A synaptic trek to autism. Curr Opin Neurobiol 19(2):231–234
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77(1):71–94
Brown RG, Marsden CD (1990) Cognitive function in Parkinson’s disease: from description to theory. Trends Neurosci 13(1):21–29
Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ (1983) A primate model of Parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA 80(14):4546–4550
Calahorro F (2011) Genetics of autism: Caenorhabditis elegans as an experimental tool in the study of the neuronal synaptic function. Ph. D. thesis, Universidad de Córdoba, Córdoba
Calahorro F, Alejandre E, Ruiz-Rubio M (2009) Osmotic avoidance in Caenorhabditis elegans: synaptic function of two genes, orthologues of human NRXN1 and NLGN1, as candidates for autism. J Vis Exp 34:e-1616. doi: 10.3791/1616. http://www.jove.com/Details.stp?ID=1616
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP et al (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415(6867):92–96
Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M (2010) Enhanced neuronal RNAi in C. elegans using SID-1. Nat Methods 7(7):554–559
Cao S, Gelwix CC, Caldwell KA, Caldwell GA (2005) Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci 25(15):3801–3812
Centre for Disease Control and Prevention (2009) Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, United States, 2006. MMWR Surveill Summ 58(10):1–20
Chalfie M, White J (1986) The nervous system. In: Wood WB (ed) The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 337–391
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S et al (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364(9440):1167–1169
Conforti L, Adalbert R, Coleman MP (2007) Neuronal death: where does the end begin? Trends Neurosci 30(4):159–166
Cuervo AM, Wong ES, Martinez-Vicente M (2010) Protein degradation, aggregation, and misfolding. Mov Disord 25(Suppl 1):S49–S54
Dauer W, Ho CC (2010) The biology and pathology of the familial Parkinson’s disease protein LRRK2. Mov Disord 25(Suppl 1):S40–S43
Dawson TM, Ko HS, Dawson VL (2010) Genetic animal models of Parkinson’s disease. Neuron 66(5):646–661
De Strooper B, Annaert W (2000) Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci 113(Pt 11):1857–1870
Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E et al (2003) Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci 6(7):708–716
DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA et al (2008) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65(8):1074–1080
Diomede L, Cassata G, Fiordaliso F, Salio M, Ami D, Natalello A et al (2010) Tetracycline and its analogues protect Caenorhabditis elegans from beta amyloid-induced toxicity by targeting oligomers. Neurobiol Dis 40(2):424–431
Dosanjh LE, Brown MK, Rao G, Link CD, Luo Y (2010) Behavioral phenotyping of a transgenic Caenorhabditis elegans expressing neuronal amyloid-beta. J Alzheimers Dis 19(2):681–690
Dostal V, Roberts CM, Link CD (2010) Genetic mechanisms of coffee extract protection in a Caenorhabditis elegans model of beta-amyloid peptide toxicity. Genetics 186(3):857–866
Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F et al (2007) Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39(1):25–27
Eapen V (2011) Genetic basis of autism: is there a way forward? Curr Opin Psychiatry 24(3):226–236
Fay DS, Fluet A, Johnson CJ, Link CD (1998) In vivo aggregation of beta-amyloid peptide variants. J Neurochem 71(4):1616–1625
Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K et al (2008) GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57(3):353–363
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391(6669):806–811
Folstein S, Rutter M (1977) Genetic influences and infantile autism. Nature 265(5596):726–728
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55(3):259–272
Garber K (2007) Neuroscience. Autism’s cause may reside in abnormalities at the synapse. Science 317(5835):190–191
Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119(7):1013–1026
Guthrie CR, Schellenberg GD, Kraemer BC (2009) SUT-2 potentiates tau-induced neurotoxicity in Caenorhabditis elegans. Hum Mol Genet 18(10):1825–1838
Haklai-Topper L, Soutschek J, Sabanay H, Scheel J, Hobert O, Peles E (2011) The neurexin superfamily of Caenorhabditis elegans. Gene Expr Patterns 11(1–2):144–150
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry (advanced online publication)
Hamamichi S, Rivas RN, Knight AL, Cao S, Caldwell KA, Caldwell GA (2008) Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson’s disease model. Proc Natl Acad Sci USA 105(2):728–733
Hattori N, Kitada T, Matsumine H, Asakawa S, Yamamura Y, Yoshino H et al (1998) Molecular genetic analysis of a novel Parkin gene in Japanese families with autosomal recessive juvenile parkinsonism: evidence for variable homozygous deletions in the Parkin gene in affected individuals. Ann Neurol 44(6):935–941
Hedgecock EM, Sulston JE, Thomson JN (1983) Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science 220(4603):1277–1279
Hunter JW, Mullen GP, McManus JR, Heatherly JM, Duke A, Rand JB (2010) Neuroligin-deficient mutants of C. elegans have sensory processing deficits and are hypersensitive to oxidative stress and mercury toxicity. Dis Model Mech 3(5–6):366–376
Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C et al (1995) Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell 81(3):435–443
Jadiya P, Chatterjee M, Sammi SR, Kaur S, Palit G, Nazir A (2011) Sir-2.1 modulates ‘calorie-restriction-mediated’ prevention of neurodegeneration in Caenorhabditis elegans: implications for Parkinson’s disease. Biochem Biophys Res Commun 413(2):306–310
Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC et al (2003) Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34(1):27–29
Jee C, Lee J, Lee JI, Lee WH, Park BJ, Yu JR et al (2004) SHN-1, a Shank homologue in C. elegans, affects defecation rhythm via the inositol-1,4,5-trisphosphate receptor. FEBS Lett 561(1–3):29–36
Jellinger KA (2001) The pathology of Parkinson’s disease. Adv Neurol 86:55–72
Kanthasamy A, Jin H, Mehrotra S, Mishra R, Rana A (2010) Novel cell death signaling pathways in neurotoxicity models of dopaminergic degeneration: relevance to oxidative stress and neuroinflammation in Parkinson’s disease. Neurotoxicology 31(5):555–561
Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y et al (2008) Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet 82(1):199–207
Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC et al (2011) Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry 168(9):904–912
Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ (2003) Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci USA 100(11):6382–6387
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S et al (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392(6676):605–608
Kraemer BC, Schellenberg GD (2007) SUT-1 enables tau-induced neurotoxicity in C. elegans. Hum Mol Genet 16(16):1959–1971
Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski JQ, Schellenberg GD (2003) Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci USA 100(17):9980–9985
Kraemer BC, Burgess JK, Chen JH, Thomas JH, Schellenberg GD (2006) Molecular pathways that influence human tau-induced pathology in Caenorhabditis elegans. Hum Mol Genet 15(9):1483–1496
Kreienkamp HJ (2008) Scaffolding proteins at the postsynaptic density: shank as the architectural framework. Handb Exp Pharmacol 186:365–380. doi:10.1007/978-3-540-72843-6_15
Kuwahara T, Koyama A, Gengyo-Ando K, Masuda M, Kowa H, Tsunoda M et al (2006) Familial Parkinson mutant alpha-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J Biol Chem 281(1):334–340
Lai CH, Chou CY, Ch’ang LY, Liu CS, Lin W (2000) Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res 10(5):703–713
Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R et al (2003) Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem 86(1):165–172
Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP et al (2004) X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet 74(3):552–557
Lawson-Yuen A, Saldivar JS, Sommer S, Picker J (2008) Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet 16(5):614–618
Levitan D, Greenwald I (1995) Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer’s disease gene. Nature 377(6547):351–354
Levy SE, Mandell DS, Schultz RT (2009) Autism. Lancet 374(9701):1627–1638
Lewy FH (1912) Paralysis agitans. Pathologische anatomie. In: Lewandowski M (ed) Handbuch der neurologie. Springer, Berlin, pp 920–933
Link CD (1995) Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA 92(20):9368–9372
Liu Z, Hamamichi S, Dae Lee B, Yang D, Ray A, Caldwell GA et al (2011) Inhibitors of LRRK2 kinase attenuate neurodegeneration and Parkinson-like phenotypes in Caenorhabditis elegans and Drosophila Parkinson’s disease models. Hum Mol Genet. doi:10.1093/hmg/ddr312
Locke CJ, Fox SA, Caldwell GA, Caldwell KA (2008) Acetaminophen attenuates dopamine neuron degeneration in animal models of Parkinson’s disease. Neurosci Lett 439(2):129–133
Mandelkow EM, Mandelkow E (1998) Tau in Alzheimer’s disease. Trends Cell Biol 8(11):425–427
Markesbery WR, Jicha GA, Liu H, Schmitt FA (2009) Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol 68(7):816–822
McColl G, Roberts BR, Gunn AP, Perez KA, Tew DJ, Masters CL et al (2009) The Caenorhabditis elegans A beta 1-42 model of Alzheimer disease predominantly expresses A beta 3-42. J Biol Chem 284(34):22697–22702
McKeith I, Cummings J (2005) Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol 4(11):735–742
McKhann GM (2011) Changing concepts of Alzheimer disease. JAMA 305(23):2458–2459
Mikolaenko I, Pletnikova O, Kawas CH, O’Brien R, Resnick SM, Crain B et al (2005) Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA). J Neuropathol Exp Neurol 64(2):156–162
Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J et al (2007) Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet 81(6):1289–1297
Moy SS, Nadler JJ (2008) Advances in behavioral genetics: mouse models of autism. Mol Psychiatry 13(1):4–26
Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J et al (1999) Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 23(3):569–582
Nam CI, Chen L (2005) Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci USA 102(17):6137–6142
Nass R, Hall DH, Miller DM 3rd, Blakely RD (2002) Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci USA 99(5):3264–3269
Oh WC, Song HO, Cho JH, Park BJ (2011) ANK repeat-domain of SHN-1 Is indispensable for in vivo SHN-1 function in C. elegans. Mol Cells 31(1):79–84
Pan T, Kondo S, Le W, Jankovic J (2009) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131:1969–1978
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276(5321):2045–2047
Pu P, Le WD (2006) C. elegans as a model system for Parkinson disease. Neurosci Bull 22(2):124–128
Saha S, Guillily MD, Ferree A, Lanceta J, Chan D, Ghosh J et al (2009) LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci 29(29):9210–9218
Sanchez B, Relova JL, Gallego R, Ben-Batalla I, Perez-Fernandez R (2009) 1,25-Dihydroxyvitamin D3 administration to 6-hydroxydopamine-lesioned rats increases glial cell line-derived neurotrophic factor and partially restores tyrosine hydroxylase expression in substantia nigra and striatum. J Neurosci Res 87(3):723–732
Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26(3):619–631
Scheiffele P, Fan J, Choih J, Fetter R, Serafini T (2000) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101(6):657–669
Schmidt E, Seifert M, Baumeister R (2007) Caenorhabditis elegans as a model system for Parkinson’s disease. Neurodegener Dis 4(2–3):199–217
Selkoe DJ (2000) The origins of Alzheimer disease: a is for amyloid. JAMA 283(12):1615–1617
Selkoe DJ, Wolfe MS (2007) Presenilin: running with scissors in the membrane. Cell 131(2):215–221
Settivari R, Levora J, Nass R (2009) The divalent metal transporter homologues SMF-1/2 mediate dopamine neuron sensitivity in Caenorhabditis elegans models of manganism and parkinson disease. J Biol Chem 284(51):35758–35768
Shastry BS (1998) Molecular genetics of familial Alzheimer disease. Am J Med Sci 315(4):266–272
Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A et al (2001) Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107(7):893–903
Silverman JL, Yang M, Lord C, Crawley JN (2010) Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11(7):490–502
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J et al (2003) Alpha-synuclein locus triplication causes Parkinson’s disease. Science 302(5646):841
Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B (1998) Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 95(13):7737–7741
Standaert DG, Yacoubian TA (2010) Target validation: the Parkinson disease perspective. Dis Model Mech 3(5–6):259–262
Starich TA, Herman RK, Kari CK, Yeh WH, Schackwitz WS, Schuyler MW et al (1995) Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics 139(1):171–188
Struhl G, Greenwald I (2001) Presenilin-mediated transmembrane cleavage is required for Notch signal transduction in Drosophila. Proc Natl Acad Sci USA 98(1):229–234
Sudhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455(7215):903–911
Sulston JE (1976) Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 275(938):287–297
Sulston JE, Brenner S (1974) The DNA of Caenorhabditis elegans. Genetics 77(1):95–104
Talebizadeh Z, Bittel DC, Veatch OJ, Butler MG, Takahashi TN, Miles JH (2004) Do known mutations in neuroligin genes (NLGN3 and NLGN4) cause autism? J Autism Dev Disord 34(6):735–736
Tan EK (2007) The role of common genetic risk variants in Parkinson disease. Clin Genet 72(5):387–393
Thomas B, Beal MF (2007) Parkinson’s disease. Hum Mol Genet 16(R2):R183–R194
Timmons L, Fire A (1998) Specific interference by ingested dsRNA. Nature 395(6705):854
Timmons L, Court DL, Fire A (2001) Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263(1–2):103–112
Turner RS, Suzuki N, Chyung AS, Younkin SG, Lee VM (1996) Amyloids beta40 and beta42 are generated intracellularly in cultured human neurons and their secretion increases with maturation. J Biol Chem 271(15):8966–8970
Turner PR, O’Connor K, Tate WP, Abraham WC (2003) Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol 70(1):1–32
Urano F, Calfon M, Yoneda T, Yun C, Kiraly M, Clark SG et al (2002) A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol 158(4):639–646
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S et al (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304(5674):1158–1160
Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K et al (2006) Neuroligins determine synapse maturation and function. Neuron 51(6):741–754
Voineskos AN, Lett TA, Lerch JP, Tiwari AK, Ameis SH, Rajji TK et al (2011) Neurexin-1 and frontal lobe white matter: an overlapping intermediate phenotype for schizophrenia and autism spectrum disorders. PLoS One 6(6):e20982
Wan L, Nie G, Zhang J, Luo Y, Zhang P, Zhang Z et al (2011) Beta-amyloid peptide increases levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radic Biol Med 50(1):122–129
Wang X, Sliwoski GR, Buttner EA (2011) The relevance of Caenorhabditis elegans genetics for understanding human psychiatric disease. Harv Rev Psychiatry 19(4):210–218
Wentzell J, Kretzschmar D (2010) Alzheimer’s disease and tauopathy studies in flies and worms. Neurobiol Dis 40(1):21–28
White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode C. elegans. Philos Trans R Soc Lond Ser B Biol Sci 314:1–340
Wiese M, Antebi A, Zheng H (2010) Intracellular trafficking and synaptic function of APL-1 in Caenorhabditis elegans. PLoS One 5(9):e12790
Wittenburg N, Eimer S, Lakowski B, Rohrig S, Rudolph C, Baumeister R (2000) Presenilin is required for proper morphology and function of neurons in C. elegans. Nature 406(6793):306–309
Wooten GF (1997) Functional anatomical and behavioral consequences of dopamine receptor stimulation. Ann NY Acad Sci 835:153–156
Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C et al (2005) Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry 10(4):329–332
Yan J, Noltner K, Feng J, Li W, Schroer R, Skinner C et al (2008) Neurexin 1alpha structural variants associated with autism. Neurosci Lett 438(3):368–370
Yanagida T, Kitamura Y, Yamane K, Takahashi K, Takata K, Yanagisawa D et al (2009) Protection against oxidative stress-induced neurodegeneration by a modulator for DJ-1, the wild-type of familial Parkinson’s disease-linked PARK7. J Pharmacol Sci 109(3):463–468
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I et al (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55(2):164–173
Zeng X, Sun M, Liu L, Chen F, Wei L, Xie W (2007) Neurexin-1 is required for synapse formation and larvae associative learning in Drosophila. FEBS Lett 581(13):2509–2516
Zoghbi HY (2003) Postnatal neurodevelopmental disorders: meeting at the synapse? Science 302(5646):826–830
Acknowledgments
We thank Prof. Lindy M. Holden-Dye for critical reading of the manuscript and for detailed comments. We also are grateful to Dr. Antonio Miranda-Vizuete for his help with the DiI dye-filling assay and valuable support. This work was supported by grant PI0197, Consejería de Salud, Junta de Andalucía.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calahorro, F., Ruiz-Rubio, M. Caenorhabditis elegans as an experimental tool for the study of complex neurological diseases: Parkinson’s disease, Alzheimer’s disease and autism spectrum disorder. Invert Neurosci 11, 73–83 (2011). https://doi.org/10.1007/s10158-011-0126-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10158-011-0126-1