Abstract
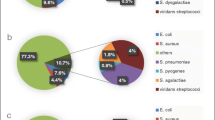
Among clinically isolated β-hemolytic streptococci, Streptococcus pyogenes and S. agalactiae were considered the main pathogens in humans until recently. In 1996, S. dysgalactiae subsp. equisimilis (SDSE) was proposed as a novel taxon among human-derived streptococcal isolates. SDSE has Lancefield group C or G antigens, exhibits strong β-hemolysis, and exerts streptokinase activity upon human plasminogen and proteolytic activity upon human fibrin. Similarly to group A streptococci, SDSE possesses virulence factors including M protein, streptolysin O, streptolysin S, streptokinase, hyaluronidase, C5a peptidase, and others. SDSE may exist among the normal flora of the skin, oropharynx, and gastrointestinal and genitourinary tracts. In the twenty-first century, invasive SDSE infection (i.e., cellulitis, urosepsis, and pneumonia) leading to various disseminated diseases is being diagnosed increasingly in Japan, elsewhere in Asia, in Europe, and in America. Particularly, among elderly patients, these invasive diseases are encountered increasingly in Japanese hospital emergency departments. Analysis of the part of the emm gene encoding the amino acid sequence at the N-terminal end of the M protein is used to determine the molecular epidemiology of SDSE. The distribution of emm types from patients with invasive or noninvasive infections differs between surveillance results from different countries. In this review, we summarize the characteristics of phenotypes and virulence factors in SDSE strains; the review also focuses on emerging SDSE infectious disease and future vaccination research.





Similar content being viewed by others
References
Wajima T, Murayama SY, Sunaoshi K, Nakayama E, Sunakawa K, Ubukata K. Distribution of emm type and antibiotic susceptibility of group A streptococci causing invasive and noninvasive disease. J Med Microbiol. 2008;57:1383–8.
Murayama SY, Seki C, Sakata H, Sunaoshi K, Nakayama E, Iwata S, et al. Capsular type and antibiotic resistance in Streptococcus agalactiae isolates from patients, ranging from newborns to the elderly, with invasive infections. Antimicrob Agents Chemother. 2009;53:2650–3.
Takahashi T, Sunaoshi K, Sunakawa K, Fujishima S, Watanabe H, Ubukata K. Clinical aspects of invasive infections with Streptococcus dysgalactiae ssp. equisimilis in Japan: differences with respect to Streptococcus pyogenes and Streptococcus agalactiae infections. Clin Microbiol Infect. doi:10.1111/j.1469-0691.2009.03047.x.
Vandamme P, Pot B, Falsen E, Kersters K, Devriese LA. Taxonomic study of Lancefield streptococcal groups C, G, and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int J Syst Bacteriol. 1996;46:774–81.
Broyles LN, Van Beneden C, Beall B, Facklam R, Shewmaker PL, Malpiedi P, et al. Population-based study of invasive disease due to β-hemolytic streptococci of groups other than A and B. Clin Infect Dis. 2009;48:706–12.
Liao CH, Liu LC, Huang YT, Teng LJ, Hsueh PR. Bacteremia caused by group G streptococci, Taiwan. Emerg Infect Dis. 2008;14:837–40.
Cohen-Poradosu R, Jaffe J, Lavi D, Grisariu-Greenzaid S, Nir-Paz R, Valinsky L, et al. Group G streptococcal bacteremia in Jerusalem. Emerg Infect Dis. 2004;10:1455–60.
Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112:622–6.
Brandt C, Spellerberg B. Streptococcus. In: Murray PR, Baron EJ, Landry ML, Jorgensen JH, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Washington: American Society for Microbiology; 2007. p. 412–29.
Lancefield RC. A serological differentiation of human and other groups of hemolytic streptococci. J Exp Med. 1933;57:571–95.
Garvie EI, Farrow JAE, Collins MD. Streptococcus dysgalactiae (Diernhofer) nom. rev. Int J Syst Bacteriol. 1983;33:404–5.
Carmeli Y, Ruoff KL. Report of cases of and taxonomic considerations for large-colony-forming Lancefield group C streptococcal bacteremia. J Clin Microbiol. 1995;33:2114–7.
Devriese LA. Streptococcal ecovars associated with different animal species: epidemiological significance of serogroups and biotypes. J Appl Bacteriol. 1991;71:478–83.
Devriese LA, Hommez J, Kilpper-Bälz R, Schleifer KH. Streptococcus canis sp. nov.: a species of group G streptococci from animals. Int J Syst Bacteriol. 1986;36:422–5.
Farrow JAE, Collins MD. Taxonomic studies on streptococci of serological groups C, G, and L and possibly related taxa. Syst Appl Microbiol. 1984;5:483–93.
Brandt CM, Haase G, Schnitzler N, Zbinden R, Lütticken R. Characterization of blood culture isolates of Streptococcus dysgalactiae subsp. equisimilis possessing Lancefield’s group A antigen. J Clin Microbiol. 1999;37:4194–7.
Katsukawa C, Tamaru A, Morikawa Y. Streptococcus dysgalactiae subsp. equisimilis possessing Lancefield’s group A antigen. Kansenshogaku Zasshi. 2002;76:155–60.
Mitsuno N, Hari T, Tamagawa N, Itoi J, Ikeda E, Hamasaki K, et al. Evaluation of rapid diagnostic kits for the detection of group A streptococcus to Streptococcus pyogenes and Streptococcus spp. with Lancefield’s group A antigen. Kansenshogaku Zasshi. 2006;80:665–73.
Tanaka D, Isobe J, Watahiki M, Nagai Y, Katsukawa C, Kawahara R, et al. Genetic features of clinical isolates of Streptococcus dysgalactiae subsp. equisimilis possessing Lancefield’s group A antigen. J Clin Microbiol. 2008;46:1526–9.
Dierksen KP, Tagg JR. Haemolysin-deficient variants of Streptococcus pyogenes and S. dysgalactiae subsp. equisimilis may be overlooked as aetiological agents of pharyngitis. J Med Microbiol. 2000;49:811–6.
Woo PC, Teng JL, Lau SK, Lum PN, Leung KW, Wong KL, et al. Analysis of a viridans group strain reveals a case of bacteremia due to lancefield group G alpha-hemolytic Streptococcus dysgalactiae subsp. equisimilis in a patient with pyomyositis and reactive arthritis. J Clin Microbiol. 2003;41:613–8.
Horii T, Izumida S, Takeuchi K, Tada T, Ishikawa J, Tsuboi K. Acute peritonitis and salpingitis associated with streptococcal toxic shock syndrome caused by Lancefield group G alpha-haemolytic Streptococcus dysgalactiae subsp. equisimilis. J Med Microbiol. 2006;55:953–6.
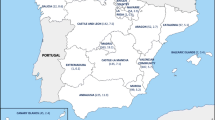
Takahashi T, Asami R, Tanabe K, Hirono Y, Nozawa Y, Chiba N, et al. Clinical aspects of invasive infection with Streptococcus dysgalactiae subsp. equisimilis in elderly patients. J Infect Chemother. 2010;16:68–71.
Kalia A, Bessen DE. Natural selection and evolution of streptococcal virulence genes involved in tissue-specific adaptations. J Bacteriol. 2004;186:110–21.
Humar D, Datta V, Bast DJ, Beall B, De Azavedo JC, Nizet V. Streptolysin S and necrotising infections produced by group G streptococcus. Lancet. 2002;359:124–9.
Hashikawa S, Iinuma Y, Furushita M, Ohkura T, Nada T, Torii K, et al. Characterization of group C and G streptococcal strains that cause streptococcal toxic shock syndrome. J Clin Microbiol. 2004;42:186–92.
Geyer A, Schmidt KH. Genetic organisation of the M protein region in human isolates of group C and G streptococci: two types of multigene regulator-like (mgrC) regions. Mol Gen Genet. 2000;262:965–76.
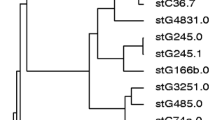
Sunaoshi K, Aburahashi H, Kobayashi R, Yamamoto Y, Okuzumi K, Yoshida A, et al. Emm typing by genetic identification of Streptococcus dysgalactiae subsp. equisimilis and susceptibility to oral antibiotics. Kansenshogaku Zasshi. 2006;80:488–95.
Sunaoshi K, Murayama SY, Adachi K, Yagoshi M, Okuzumi K, Chiba N, et al. Molecular emm genotyping and antibiotic susceptibility of Streptococcus dysgalactiae subsp. equisimilis isolated from invasive and non-invasive infections. J Med Microbiol. 2010;59:82–8.
Ikebe T, Murayama S, Saitoh K, Yamai S, Suzuki R, Isobe J, et al. Surveillance of severe invasive group-G streptococcal infections and molecular typing of the isolates in Japan. Epidemiol Infect. 2004;132:145–9.
Ahmad Y, Gertz RE Jr, Li Z, Sakota V, Broyles LN, Van Beneden C, et al. Genetic relationships deduced from emm and multilocus sequence typing of invasive Streptococcus dysgalactiae subsp. equisimilis and S. canis recovered from isolates collected in the United States. J Clin Microbiol. 2009;47:2046–54.
Misawa Y, Okugawa S, Ubukata K, Okuzumi K, Okada M, Moriya K, et al. A case of severe necrotizing cellulitis caused by group G Streptococcus dysgalactiae subsp. equisimilis. Kansenshogaku Zasshi. 2006;80:436–9.
Ueno K, Kawayama T, Edakuni N, Koga T, Aizawa H. A case of thoracic empyema with gas formation associated with Streptococcus dysgalactiae subsp. equisimilis. Kansenshogaku Zasshi. 2006;80:527–30.
Matsui D, Kitasato Y, Honda S, Ueno K, Tanaka A, Edakuni N, et al. A case of bacterial pneumonia caused by Streptococcus dysgalactiae subsp. equisimilis, showing patchy consolidations resembling organizing pneumonia. Nihon Kokyuki Gakkai Zasshi. 2007;45:36–42.
Horibe M, Sano Y, Himeno T, Ichikawa M, Ban Y, Kano H, et al. Case of gas gangrene in both legs due to Streptococcus dysgalactiae subsp. equisimilis, resulting in amputation of right leg. Nippon Naika Gakkai Zasshi. 2008;97:1879–81.
Bramhachari PV, Kaul SY, McMillan DJ, Shaila MS, Karmarkar MG, Sriprakash KS. Disease burden due to Streptococcus dysgalactiae subsp. equisimilis (group G and C streptococcus) is higher than that due to Streptococcus pyogenes among Mumbai school children. J Med Microbiol. 2010;59:220–3.
Sing A, Trebesius K, Heesemann J. Diagnosis of Streptococcus dysgalactiae subspecies equisimilis (Group C streptococci) associated with deep soft tissue infections using fluorescent in situ hybridization. Eur J Clin Microbiol Infect Dis. 2001;20:146–9.
Kumar A, Sandoe J, Kumar N. Three cases of vertebral osteomyelitis caused by Streptococcus dysgalactiae subsp. equisimilis. J Med Microbiol. 2005;54:1103–5.
Pinho MD, Melo-Cristino J, Ramirez M. Clonal relationships between invasive and noninvasive Lancefield group C and G streptococci and emm-specific differences in invasiveness. J Clin Microbiol. 2006;44:841–6.
Siljander T, Karppelin M, Vähäkuopus S, Syrjänen J, Toropainen M, Kere J, et al. Acute bacterial, nonnecrotizing cellulitis in Finland: microbiological findings. Clin Infect Dis. 2008;46:855–61.
Fernández-Martínez AI, Pascual MR, Cimas D, Esteban J. Septic arthritis due to Streptococcus dysgalactiae ssp. equisimilis. Enferm Infecc Microbiol Clin. 2008;26:670–1.
Lestin F, Mann S, Podbielski A. Spondylodiscitis and paraspinal abscess caused by beta-haemolytic group G streptococci spreading from infected leg ulcers. J Med Microbiol. 2008;57:1157–60.
Kittang BR, Langeland N, Skrede S, Mylvaganam H. Two unusual cases of severe soft tissue infection caused by Streptococcus dysgalactiae subsp. equisimilis. J Clin Microbiol. 2010;48:1484–7.
Lopardo HA, Vidal P, Sparo M, Jeric P, Centron D, Facklam RR, et al. Six-month multicenter study on invasive infections due to Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis in Argentina. J Clin Microbiol. 2005;43:802–7.
Woo PC, Fung AM, Lau SK, Wong SS, Yuen KY. Group G beta-hemolytic streptococcal bacteremia characterized by 16S ribosomal RNA gene sequencing. J Clin Microbiol. 2001;39:3147–55.
Takahashi T, Yoshino M, Morozumi M, Chiba N, Kishii K, Murayama SY, Ubukata K. Clinical aspects of invasive infection with Lancefield group B and G streptococci in elderly patients. Kansenshogaku Zasshi. 2010;84:135.
Yamaoka S, Ogihara T, Yasui M, Hasegawa M, Hira S, Oue S, et al. Neonatal streptococcal toxic shock syndrome caused by Streptococcus dysgalactiae subsp. equisimilis. Pediatr Infect Dis J. 2010. doi:10.1097/INF.0b013e3181e5292f.
Kittang BR, Langeland N, Mylvaganam H. Distribution of emm types, subtypes among noninvasive group A, C and G streptococcal isolates in western Norway. APMIS. 2008;116:457–64.
Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997–2004). Diagn Microbiol Infect Dis. 2006;55:119–27.
Pinho MD, Melo-Cristino J, Ramirez M. Fluoroquinolone resistance in Streptococcus dysgalactiae subsp. equisimilis and evidence for a shared global gene pool with Streptoccocus pyogenes. Antimicrob Agents Chemother. doi:10.1128/AAC.01377-09.
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: seventeenth informational supplement. CLSI document M100-S17. Wayne, PA: CLSI; 2007.
Liu LC, Tsai JC, Hsueh PR, Tseng SP, Hung WC, Chen HJ, et al. Identification of tet(S) gene area in tetracycline-resistant Streptococcus dysgalactiae subsp. equisimilis clinical isolates. J Antimicrob Chemother. 2008;61:453–5.
Uh Y, Hwang GY, Jang IH, Cho HM, Noh SM, Kim HY, et al. Macrolide resistance trends in beta-hemolytic streptococci in a tertiary Korean hospital. Yonsei Med J. 2007;48:773–8.
Savini V, Catavitello C, Talia M, Manna A, Pompetti F, Di Bonaventura G, et al. β-Lactam failure in treatment of two group G Streptococcus dysgalactiae subsp. equisimilis pharyngitis patients. J Clin Microbiol. 2008;46:814–6.
Lessa F, Leparc GF, Benson K, Sanderson R, Van Beneden CA, Shewmaker PL, et al. Fatal group C streptococcal infection due to transfusion of a bacterially contaminated pooled platelet unit despite routine bacterial culture screening. Transfusion. 2008;48:2177–83.
Bay JO, Tournilhac O, Ducher E, Romaszko JP, Ergani A, Bouvet A, et al. A near fatal septic transfusion reaction due to Streptococcus dysgalactiae subspecies equisimilis calls for novel safety measures. Vox Sang. 2009;96:271.
Courbil R, Romaszko JP, Odent-Malaure H, Fabrigli P, Chavarin P, Tournilhac O, et al. Analysis of a severe septic transfusion reaction with standard platelet concentrate. Transfus Clin Biol. 2010;17:9–13.
Kawata K, Minakami T, Mori Y, Katsumi M, Kataoka Y, Ezawa A, et al. rDNA sequence analyses of Streptococcus dysgalactiae subsp. equisimilis isolates from pigs. Int J Syst Evol Microbiol. 2003;53:1941–6.
Laus F, Preziuso S, Spaterna A, Beribè F, Tesei B, Cuteri V. Clinical and epidemiological investigation of chronic upper respiratory diseases caused by beta-haemolytic Streptococci in horses. Comp Immunol Microbiol Infect Dis. 2007;30:247–60.
Imai D, Jang S, Miller M, Conrad PA. Characterization of beta-hemolytic streptococci isolated from southern sea otters (Enhydra lutris nereis) stranded along the California coast. Vet Microbiol. 2009;136:378–81.
Zoric M, Nilsson E, Lundeheim N, Wallgren P. Incidence of lameness and abrasions in piglets in identical farrowing pens with four different types of floor. Acta Vet Scand. 2009;51:23.
Zaoutis T, Attia M, Gross R, Klein J. The role of group C and group G streptococci in acute pharyngitis in children. Clin Microbiol Infect. 2004;10:37–40.
Smeesters PR, Mardulyn P, Vergison A, Leplae R, Van Melderen L. Genetic diversity of Group A Streptococcus M protein: implications for typing and vaccine development. Vaccine. 2008;26:5835–42.
Dale JB, Penfound T, Chiang EY, Long V, Shulman ST, Beall B. Multivalent group A streptococcal vaccine elicits bactericidal antibodies against variant M subtypes. Clin Diagn Lab Immunol. 2005;12:833–6.
Hu MC, Walls MA, Stroop SD, Reddish MA, Beall B, Dale JB. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect Immun. 2002;70:2171–7.
McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, et al. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41:1114–22.
Nir-Paz R, Korenman Z, Ron M, Michael-Gayego A, Cohen-Poradosu R, Valinsky L, et al. Streptococcus pyogenes emm and T types within a decade, 1996–2005: implications for epidemiology and future vaccines. Epidemiol Infect. 2010;138:53–60.
Guilherme L, Faé KC, Higa F, Chaves L, Oshiro SE, Freschi de Barros S, et al. Towards a vaccine against rheumatic fever. Clin Dev Immunol. 2006;13:125–32.
Vohra H, Dey N, Gupta S, Sharma AK, Kumar R, McMillan D, et al. M protein conserved region antibodies opsonise multiple strains of Streptococcus pyogenes with sequence variations in C-repeats. Res Microbiol. 2005;156:575–82.
Yoonim N, Olive C, Pruksachatkunakorn C, Pruksakorn S. Bactericidal activity of M protein conserved region antibodies against group A streptococcal isolates from the Northern Thai population. BMC Microbiol. 2006;6:71.
Acknowledgments
Our study was funded in part by a grant under the category, ‘‘Research on Emerging and Re-emerging Infectious Diseases’’ (H19-002 and H22-013), from the Japanese Ministry of Health, Labour and Welfare (to Drs. K. Ubukata and H. Watanabe). The authors thank Katsuhiko Sunaoshi, Keisuke Okada, and Akiko Ono for assistance with manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takahashi, T., Ubukata, K. & Watanabe, H. Invasive infection caused by Streptococcus dysgalactiae subsp. equisimilis: characteristics of strains and clinical features. J Infect Chemother 17, 1–10 (2011). https://doi.org/10.1007/s10156-010-0084-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-010-0084-2