Abstract
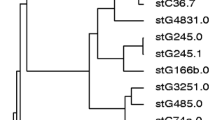
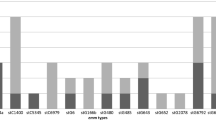
Prevalence of invasive ß-haemolytic streptococci (BHS) at a tertiary care hospital and molecular diversity of S. pyogenes and S. dysgalactiae was studied. Between 2012 and 2016, all blood culture sets (n = 55,839), CSF (n = 8413) and soft tissue (n = 20,926) samples were analysed for BHS positivity using HYBASE software. Molecular profiles of 99 S. pyogenes and S. dysgalactiae were identified by sequencing of M protein genes (emm types) and multiplex PCR typing of 20 other virulence determinants. Streptococci contributed to 6.2% of blood, 10.7% of CSF and 14.5% of soft tissue isolates, being among the most common invasive isolates. The overall rates of invasive S. pyogenes, S. agalactiae, S. dysgalactiae and S. pneumoniae were 2.4, 4.4, 2.1, and 5.3%. Whereas S. pneumoniae was 1.5% more common in CSF samples, BHS isolates were 2-fold and 11-fold higher in bacteraemia and invasive soft tissue infections. Genetic BHS typing revealed wide molecular diversity of invasive and noninvasive group A and group G BHS, whereas one emm-type (stG62647.0) and no other virulence determinants except scpA were detected in invasive group C BHS. BHS were important invasive pathogens, outpacing S. pneumoniae in bacteraemia and invasive soft tissue infections. The incidence of S. dysgalactiae infections was comparable to that of S. pyogenes even with less diversity of molecular virulence. The results of this study emphasise the need for awareness of BHS invasiveness in humans and the need to develop BHS prevention strategies.




Similar content being viewed by others
References
Thompson CC, Emmel VE, Fonseca EL et al (2013) Streptococcal taxonomy based on genome sequence analyses. F1000Research. https://doi.org/10.3410/f1000research.2-67.v1
Rantala S (2014) Streptococcus dysgalactiae subsp. equisimilis bacteremia: an emerging infection. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-014-2092-0
Oppegaard O, Mylvaganam H, Kittang BR (2015) Beta-haemolytic group A, C and G streptococcal infections in Western Norway: a 15-year retrospective survey. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2014.08.019
Wong SS, Lin YS, Mathew L et al (2009) Increase in group G streptococcal infections in a community hospital, New York, USA. Emerg Infect Dis. https://doi.org/10.3201/eid1506.080666
Bramhachari PV, Kaul SY, McMillan DJ et al (2010) Disease burden due to Streptococcus dysgalactiae subsp. equisimilis (group G and C streptococcus) is higher than that due to Streptococcus pyogenes among Mumbai school children. J Med Microbiol. https://doi.org/10.1099/jmm.0.015644-0
Bruun T, Kittang BR, de Hoog BJ, et al (2013) Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin Microbiol Infect. https://doi.org/10.1111/1469-0691.12276
Hashikawa S, Iinuma Y, Ohkura T et al (2004) Characterization of group C and G streptococcal strains that cause streptococcal toxic shock syndrome. J Clin Microbiol. https://doi.org/10.1128/JCM.42.1.186
Brandt CM, Schweizer KG, Holland R et al (2005) Lack of mitogenic activity of speG- and speG(dys)-positive Streptococcus dysgalactiae subspecies equisimilis isolates from patients with invasive infections. Int J Med Microbiol. https://doi.org/10.1016/j.ijmm.2005.07.013
Babbar A, Itzek A, Pieper DH, Nitsche-Schmitz DP (2018) Detection of Streptococcus pyogenes virulence genes in Streptococcus dysgalactiae subsp. equisimilis from Vellore, India. Folia Microbiol. https://doi.org/10.1007/s12223-018-0595-2
Gherardi G, Imperi M, Palmieri C et al (2014) Genetic diversity and virulence properties of Streptococcus dysgalactiae subsp. equisimilis from different sources. J Med Microbiol. https://doi.org/10.1099/jmm.0.062109-0
Lo H-H, Cheng W-S (2015) Distribution of virulence factors and association with emm polymorphism or isolation site among beta-hemolytic group G Streptococcus dysgalactiae subspecies equisimilis. APMIS. https://doi.org/10.1111/apm.12305
Center for Disease Control and Prevention (CDC) (2015) Streptococcus laboratory—protocol for emm typing. http://www.cdc.gov/streplab/protocol-emm-type.html. Accessed 29 Sep 2017
Sanderson-Smith M, De Oliveira DMP, Guglielmini J et al (2014) A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J Infect Dis. https://doi.org/10.1093/infdis/jiu260
Borek AL, Wilemska J, Izdebski R, et al (2011) A new rapid and cost-effective method for detection of phages, ICEs and virulence factors encoded by Streptococcus pyogenes. Pol J Microbiol
Simpson EH (1949) Measurement of diversity. Nature. https://doi.org/10.1038/163688a0
Grundmann H, Hori S, Tanner G (2001) Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J Clin Microbiol. https://doi.org/10.1128/JCM.39.11.4190-4192.2001
Weinstein MP, Towns ML, Quartey SM et al (1997) The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. https://doi.org/10.1093/clind/24.4.584
Hall KK, Lyman JA (2006) Updated review of blood culture contamination. Clin Microbiol Rev. https://doi.org/10.1128/CMR.00062-05
Karakullukçu A, Kuşkucu MA, Ergin S et al (2017) Determination of clinical significance of coagulase-negative staphylococci in blood cultures. Diagn Microbiol Infect Dis. https://doi.org/10.1016/j.diagmicrobio.2016.12.006
Khatib R, Labalo V, Sharma M et al (2017) Enterococcus spp. in a single blood culture: bacteraemia or contamination? Diagn Microbiol Infect Dis. https://doi.org/10.1016/j.diagmicrobio.2016.12.005
Freeman JT, Chen LF, Sexton DJ, Anderson DJ (2011) Blood culture contamination with Enterococci and skin organisms: implications for surveillance definitions of primary bloodstream infections. Am J Infect Control. https://doi.org/10.1016/j.ajic.2010.07.014
Weinstein MP (2003) Blood culture contamination: persisting problems and partial progress. J Clin Microbiol. https://doi.org/10.1128/JCM.41.6.2275
Seifert H, Abele-Horn M, Fätkenheuer G et. al (2007) Mikrobiologisch-infektiologische Qualitätsstandards (MiQ). Qualitätsstandards in der mikrobiologischen-infektiologischen Diagnostik. Blutkulturdiagnostik Sepsis, Endokarditis, Katheterinfektionen, Teil I. ELSEVIER-Urban & Fischer, München, Jena
Public Health England (2014) Investigation of blood cultures (for organisms other than Mycobacterium species). UK Standards for Microbiology Investigations B37 Issue 8
Public Health England (2014) Meningoencephalitis. UK Standards for Microbiology Investigations S5 Issue 1
Moore CE, Paul J, Foster D et al (2014) Reduction of invasive pneumococcal disease 3 years after the introduction of the 13-valent conjugate vaccine in the Oxfordshire region of England. J Infect Dis. https://doi.org/10.1093/infdis/jiu213
Rodriguez-Granger J, Alvargonzalez JC, Berardi A et al (2012) Prevention of group B streptococcal neonatal disease revisited. The DEVANI European project. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-012-1559-0
Sendi P, Johansson L, Norrby-Teglund A (2008) Invasive group B streptococcal disease in non-pregnant adults: a review with emphasis on skin and soft-tissue infections. Infection. https://doi.org/10.1007/s15010-007-7251-0
Rantala S, Vuopio-Varkila J, Vuento R et al (2009) Clinical presentations and epidemiology of β-haemolytic streptococcal bacteraemia: a population-based study. Clin Microbiol Infect. https://doi.org/10.1111/j.1469-0691.2008.02672.x
Laupland KB, Kibsey PC, Gregson DB, Galbraith JC (2013) Population-based laboratory assessment of the burden of community-onset bloodstream infection in Victoria, Canada. Epidemiol Infect. https://doi.org/10.1017/S0950268812000428
Heyderman RS, Madhi SA, French N et al (2016) Group B streptococcus vaccination in pregnant women with or without HIV in Africa: a non-randomised phase 2, open-label, multicentre trial. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(15)00484-3
Center for Disease Control and Prevention (CDC) (2015) Surveillance reports. https://www.cdc.gov/abcs/reports-findings/surv-reports.html. Accessed 29 Sep 2017
Cohen D, Ferne M, Rouach T, Bergner-Rabinowitz S (1987) Food-borne outbreak of group G streptococcal sore throat in an Israeli military base. Epidemiol Infect. https://doi.org/10.1017/S0950268800067716
Yamaguchi T, Kawahara R, Katsukawa C et al (2018) Food-borne outbreak of group G streptococcal pharyngitis in a school dormitory in Osaka, Japan. J Clin Microbiol. https://doi.org/10.1128/JCM.01884-17
Kittang BR, Skrede S, Langeland N et al (2011) emm gene diversity, superantigen gene profiles and presence of SlaA among clinical isolates of group A, C and G streptococci from western Norway. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-010-1105-x
Wajima T, Morozumi M, Hanada S et al (2016) Molecular characterization of invasive Streptococcus dysgalactiae subsp. equisimilis, Japan. Emerg Infect Dis. https://doi.org/10.3201/eid2202.141732
McNeilly CL, McMillan DJ (2014) Horizontal gene transfer and recombination in Streptococcus dysgalactiae subsp. equisimilis. Front Microbiol. https://doi.org/10.3389/fmicb.2014.00676
Lefébure T, Richards VP, Lang P et al (2012) Gene repertoire evolution of Streptococcus pyogenes inferred from phylogenomic analysis with Streptococcus canis and Streptococcus dysgalactiae. PLoS One. https://doi.org/10.1371/journal.pone.0037607
Beres SB, Musser JM (2007) Contribution of exogenous genetic elements to the group a Streptococcus metagenome. PLoS One. https://doi.org/10.1371/journal.pone.0000800
Acknowledgements
We thank Uta Falke and Leonhard Menschner for technical support and Patric Nitsche-Schmitz for providing the BHS reference strains.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethic approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
ESM 1
(DOCX 811 kb)
Rights and permissions
About this article
Cite this article
Rößler, S., Berner, R., Jacobs, E. et al. Prevalence and molecular diversity of invasive Streptococcus dysgalactiae and Streptococcus pyogenes in a German tertiary care medical centre. Eur J Clin Microbiol Infect Dis 37, 1325–1332 (2018). https://doi.org/10.1007/s10096-018-3254-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3254-2