Abstract
Beta-hemolytic group G streptococci (GGS) are increasingly recognized as a source of substantial morbidity, causing mild to severe sporadic infections as well as outbreaks. The purpose of this study was to determine the genetic diversity and antibiotic resistance of GGS in Israel in order to aid in prevention and control. A total of 325 GGS isolates were collected in Israel between 2007 and 2011 from three determined settings: (1) carriage (n = 60), an observational longitudinal carriage study in the IF, (2) non-invasive (n = 166), clinical sporadic and epidemic non-invasive cases in the IDF, and (3) invasive (n = 99) cases of bacteremia collected during this period in Israel from a similar age group, at the national Streptococcal Reference Center. All isolates were characterized genetically and by their antibiotic-resistance profile. emm typing revealed 35 distinct types and subtypes among 228 S. dysgalactiae subsp. equisimilis (SDSE) isolates, with high genetic diversity. An additional 97 GGS were identified as Streptococcus anginosus (SAG). The proportion of SDSE was higher in the invasive (100 %) and non-invasive (63.8 %) isolates compared to the carriage ones (38.3 %). Clindamycin, erythromycin, azithromycin and tetracycline resistance was detected in 6.6 %, 8.6 %, 9.7 % and 37.6 % of isolates, respectively. Overall, the most resistant isolates were in the invasive group and the fewest were in the SAG group. Considerable genetic diversity and common antibiotic resistance were revealed among GGS strains which differed according to the epidemiologic settings. Further clinical, epidemiological and basic research of GGS as a pathogen is warranted.
Similar content being viewed by others
Introduction
Beta-hemolytic streptococci are widely spread human pathogens possessing Lancefield group A, B, C, G, F and L antigens. Group G streptococci (GGS) comprise a variety of species that are part of the normal microbial flora of the upper respiratory tract, skin, gut and female genital tract. Streptococcus dysgalactiae subsp. equisimilis (SDSE) isolates cause a variety of mild to moderate infections in humans, including those of the pharynx, skin and soft-tissue, as well as severe infections, such as bacteremia and sepsis. Other streptococcal species that are pathogenic in humans are classified as the Streptococcus anginosus group (SAG) [1], which consist of S. anginosus, S. constellatus subspecies constellatus, S. constellatus subsp. pharyngis, and S. intermedius. SAG isolates were identified as the most frequent cause of invasive pyrogenic streptococcal infection in Canada [2]. The SAG group is easily distinguishable from the SDSE group by biologic characteristics and colony size on sheep blood agar plates [3].
The association of group G streptococci with outbreaks and severe invasive disease has been previously reported [3–5]. The burden of invasive disease caused by SDSE is described as being comparable to that caused by invasive group A streptococcal (GAS) emm types, and affects similar adult populations [6]. GGS was reportedly found more commonly in the throat than GAS among tropical communities in Northern Australia [7].
Human-associated strains of GGS harbor virulence factors similar to those expressed by GAS, including the M protein, the major virulence factor of both these important pathogens. The M protein is a key virulence factor that acts as an adhesin and invasin, conferring resistance to phagocytosis [1, 3]. Analysis of the 5′ of the emm gene encoding the amino acid sequence at the N terminal end of the M protein is used for molecular epidemiology. This sequence-based typing scheme developed for GAS has also been successfully applied to GGS and group C streptococci (GCS) SDSE isolates (http://www.cdc.gov/ncidod/biotech/strep), with over 73 known emm types and 160 identified SDSE subtypes.
In the last decade, as a result of improved detection methods, increased virulence, and expanding populations of compromised hosts [5], GGS/GCS strains have been frequently found to be the cause of mild to severe infections [8]. However, there are sparse data on the molecular characterization GGS in different epidemiologic settings.
Sporadic and epidemic illness caused by streptococci is a significant public health problem in the Israel Defense Forces (IDF) [9, 10]. Pharyngitis and cutaneous outbreaks, several of which are caused by GGS, occur several times a year in combat and other military units. Invasive GGS infections are an emerging problem in Israel as in other industrial countries [4, 5]. The burden of invasive disease caused by SDSE is comparable to that caused by invasive S. pyogenes and affects similar adult populations [11]. In this study, we determined the molecular diversity of GGS in Israel. Specifically, we identified the molecular epidemiology and antibiotic resistance profile of GGS isolates from three different epidemiologic settings in Israel: carriage, non-invasive and invasive.
Materials and methods
Study design population and setting
Isolates of GGS were collected during 2007–2011 from Israel Defense Force (IDF) soldiers, aged 18–49 (settings 1 & 2), and from bacteremia cases in Israel of patients aged 18–79 (setting 3). We analyzed isolates at the National Streptococci Reference Laboratory, obtained from three different epidemiological settings, determined a priori:
Carriage
An observational longitudinal carriage study was conducted among healthy male soldiers during the years 2007–2008. Throat samples were obtained on five occasions during 6 months of basic training. The setting is described in greater detail elsewhere [12]. All the recovered isolates of GGS were included in the present study. A single isolate was included in cases where the same emm type was isolated from one individual on several occasions. Four isolates sampled from healthy individuals during an outbreak (detailed below) were also included in this group.
Non-invasive
The isolates were derived from throat cultures ordered by treating physicians in the IDF during 2007–2011. GGS isolates identified at a large pharyngitis outbreak that occurred at 2009 were included [13]. The outbreak isolate (stC1400.9) was included once in this group.
Invasive
These were GGS isolates routinely sent by clinical laboratories in Israel to the National Streptococci Reference Laboratory during 2007–2011, as required by the Ministry of Health regulations. An invasive strain was defined as a strain that had been isolated from a normally sterile site (in this study the strains were isolated from bacteremia).
Bacterial isolation
Catalase-negative, chain-forming, gram positive cocci were Beta-hemolysis and colony size confirmed in tryptic soy supplemented with 5 % sheep blood agar (Oxoid, Hampshire, England), after overnight incubation at 37 °C. The Lancefield group was confirmed by a commercial latex agglutination technique, the SlidexStrepto-Kit (bioMérieux, Marcy-l’Etoile, France).
emm typing and SAG identification
DNA extraction and emm PCR were performed on all GGS isolates as described (http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm). PCR fragments were subjected to sequencing at the Center for Genome Technology at the Hebrew University of Jerusalem or a commercial service company. Isolates that did not yield an emm PCR result were tested for SAG using a specific PCR assay [14]. Briefly, PCR was performed with milleri-EL-F (5′-ACTCTTGTGTTAAATAAAATCC-3′), and milleri-EL-2R (5′-ACGCAGCATTTTGAAGRGCA-3′) primers of the groEL gene. An isolate with a 700 bp band was defined as SAG. The emm sequence results were submitted to the emm sequence database at the Center of Disease Control (Atlanta, GA, USA) to assign the type and subtype according to the exact match of 180 bases of the 5′ sequence. Multiplex alignment of the full sequence to observe the phylogenetic relations between isolates was performed with Bionumerics (Applied Maths, Belgium).
Antibiotic susceptibility
A total of 284 GGS available isolates were tested using the disc diffusion method (Kirby-Bauer) according to the 2011 guidelines of the Clinical and Laboratory Standards Institute (M100-S201). Discs of ampicillin (10 μg), penicillin (10 unit), ofloxacin (5 μg), ceftriaxone (30 μg), erythromycin (15 μg), clindamycin (2 μg), tetracycline (30 μg) and azithromycin (15 μg) were obtained from OXOID (United Kingdom).
Statistical analysis
Proportions of antibiotic resistance and genetic types were analyzed overall and individually by sampling each setting and each subspecies. The proportion of SDSE was compared between the sampling settings (with carriage as the reference group) by the two-tailed Fisher’s exact test. The proportions of isolates with antibiotic resistance against each antimicrobial agent were compared between the three groups (SDSE invasive, SDSE non-invasive or carriage), and the SAG non-invasive or carriage groups by the two-tailed Fisher’s exact test. A pvalue < 0.05 was considered significant. Analyses were done with WINPEPI [15].
Results
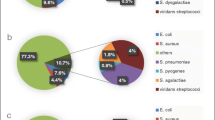
A total of 325 GGS isolates were collected and characterized: 99 invasive, 166 non-invasive and 60 carriage isolates. Species identification revealed that they were either SDSE (228, 70.1 %) or SAG (97, 29.8 %). The SDSE isolates comprised all 99 (100.0 %) of the invasive strains, 106 (63.8 %) of the non-invasive strains and 23 (38.3 %) of the carriage strains isolated during this study (Table 1).
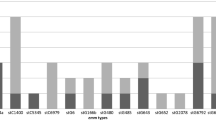
A total of 35 distinct emm types were identified among the 228 SDSE isolates, reflecting high strain diversity. The most frequent five emm types were of sequence type stG485.0 (17.5 %), followed by stG166b.0 (12.3 %), stG6.1 (9.2 %), stC1400.0 (7.9 %) and stG652.0 (5.7 %). These emm types comprised 52.6 % of the isolates, while the other 30 emm types accounted for the remainder (Table 2).
The distribution of emm types for the three settings was similar, with slight variations. The invasive emm type isolates were stG485.0 (23.2 %), stG166b.0 (16.2 %), stG6.1 (9.1 %), stG2078.0 (8.1 %) and stG652.0 (7.1 %), and they accounted for 63 % of the strains. There was only one stc1400.0 (1 %). The most frequent emm type among the non-invasive isolates was stc1400.0 (15.1 %), followed by stG485.0 (11.5 %), stG166b.0 (8.7 %), stG6.1 (8.7 %), and stG480.0 (7.7 %), comprising 50 % of the isolates. The carriage isolates were stG485.0 (14.8 %), stG166b.0 (13 %) and stG6.1 (13 %). The other 11 emm types consisted of small numbers of isolates of each emm type (2-1). The difference in the frequency of emm types of the carriage isolates can also be due to the smaller numbers of isolates in this group.
Multiple sequence alignment analysis conducted for all emm amplicons obtained from the 35 different emm types identified in this study indicated high diversity. The emm stC1400.9 that had been defined at the Streptococcus Laboratory at CDC as a new subtype was first identified during a severe and extensive pharyngitis outbreak (119 subjects) in a military unit [13]. This subtype was identified from the pharynx of 43 sick (43/119, 36 %) and five healthy (5/24, 20 %) young adults. Interestingly, SDSE isolated from four patients with tonsillitis at the outbreak were found to be genetically close, but were different emm subtypes (stC1400.0 and stC1400.2), indicating a significant nucleotide polymorphism from the Israeli outbreak strain. Therefore, we excluded the possibility of bacterial passage during a single-source outbreak (10 days) (Fig. 1).
Antibiotic testing of 290 isolates showed susceptibility to ampicillin, penicillin, ofloxacin and cefotaxime. Resistance to clindamycin, erythromycin, azithromycin and tetracycline was detected in 6.6 %, 8.6 %, 9.7 % and 37.6 % of isolates, respectively. All the tested SAG isolates (n = 75) were susceptible to erythromycin and clindamycin. Only one isolate was resistant to azithromycin, and seven were resistant to tetracycline. SDSE isolates showed significantly higher resistance to antimicrobial agents than SAG isolates (Table 3).
Discussion
Determination of the genetic diversity and antibiotic resistance of GGS can contribute key information for the epidemiology and control of disease of this species nationwide. We analyzed a collection of 325 GGS strains representing widely diverse sources and populations in Israel during a 5-year surveillance period (2007–2011). The isolates were collected from patients aged 18–79 years old. The other isolates, some of which led to outbreaks, were isolated from sick and healthy soldiers, 18–49 years old serving in the IDF.
All the GGS invasive infections were caused by SDSE, while 63.8 % of the non-invasive cases and 38.3 % of the carriage strains were SDSE. Thirty-five different emm types were identified among the 228 SDSE isolates. Overall, more than 52 % of the isolates were comprised of five emm types, indicating a high level of diversity among the SDSE isolates in Israel. The most common types of all the SDSE isolates were stG485.0, stG166b, stG6.1 and stG1400.0. The prevalence of emm types stG485.0 and stG166b was even higher in the invasive group (23.2 % and 16.2 %, respectively). These results are in accordance with other studies: emm types stG485.0 and stG6.1 were reported as being the most common types identified among patients with group G bacteremia in a hospital setting in Jerusalem [6], in Chennai, south India [16] and in a hospital setting in Northern Taiwan [17]. The total high prevalence of those emm types in all other isolates (non-invasive and carriage) indicates the likelihood of a possible reservoir for severe infections. Similarly to S. pyogenes, SDSE is transmitted through direct contact, person-to-person, from either carriage or disease isolates.
Several isolates carry emm sequences from other Streptococcus species such as S. pyogenes (emm 5.6 and 102.3) and GCS (emm stC1400.0, stC1400.2, stC1400.9, stC74a.0, stC36.0, and stC36.7), indicating the possibility of horizontal transfer of the emm genes from S. pyogenes and GCS to GGS isolates. The phylogenetic relation between SDSE and S. pyogenes alleles reveals a history of interspecies recombination, with either species often serving as genetic donors [7].
The results of a multiple sequence alignment of the full sequence of the emm amplicon from the 35 different emm types identified in this study indicated a high diversity of the sequences. Excluding the subtypes, obvious derivatives of the same type, all emm types were less than 60 % identical within the full sequence, and shared less than an 87 % identity within the 50 codons encoding the predicted N terminus of the M protein (Fig. 1). Low genetic relatedness was also found among frequent isolates (stG485.0, stG166b.0 and stG6.1).
Our study revealed emm-type diversity of SDSE isolates in Israel. SDSE infections are increasing globally. Practical implications like vaccine development and epidemiological investigations which depend on universal and standard molecular subtyping of the strains for disease preventive and control are suggested.
Our results of SDSE emm types of both invasive and non-invasive strains differed from surveillance results reported globally by several groups [11]. As such, it would appear that the emm types of both invasive and non-invasive SDSE are diverse between countries and regions, as opposed to S. pyogens which include typical (but not exclusive) invasive emm types.
Testing for susceptibility to eight antimicrobial agents on 284 GGS, SDSE and SAG isolates revealed that all the tested isolates were susceptible to cephalosporin, oflaxacin and penicillin, which are the drugs of choice for treatment. According to the literature, human GCS/GGS isolates remain almost uniformly susceptible to penicillin and other β-lactam agents [18]. Resistance to erythromycin, clindamycin and tetracycline is prevalent among GGS strains in Israel. Macrolide resistance in SDSE has also been shown to be widespread in many countries [18]. Tetracycline no longer represents an option for the empirical treatment of SDSE isolates [2].
In the present study, SAG isolates were susceptible to erythromycin and clindamycin, unlike the results of a study on SAG isolates in Germany that reported resistance to erythromycin, clindamycin and ciprofloxacin [2]. Antibiotic resistance of the non-invasive and carriage SDSE isolates was significantly higher than that of the SAG isolates. Furthermore, the invasive SDSE isolates were highly resistant to all four antibiotic agents, emphasizing the significance of these isolates. These results are in accordance with the comparative responses of the bacteria groups, i.e., non-invasive and carriage isolates versus invasive isolates, to medications.
There are several limitations to our study. Only 290 out of 325 isolates (89.2 %) were available for characterization of antibiotic resistance, due to the long storage period before testing. The invasive strains were sent from clinical laboratories of hospitals, while the carriage and non-invasive strains were isolated mostly from young individuals with no apparent underlying diseases [12]. Taking the different subjects’ characteristics between the settings, cautious interpretation of differences between the three epidemiologic settings is warranted. However, we found significant differences in proportion of SDSE between the carriage and non-invasive groups, which are taken from the same target population of young soldiers. In addition, all invasive isolates were SDSE, including isolates from subjects aged 18–49.
The present study provides insights into the pathogen’s genetic basis and disease properties shared with GAS. Further research to reveal more genetic information about the SDSE isolates is needed in order to understand the genetic variability in the SDSE population, especially the variability due to recombination involving housekeeping genes and lateral gene transfer of virulence factors from S. pyogenes.
This study is the first comprehensive analysis of molecular epidemiology and antibiotic resistance of GGS in Israel, comparing isolates derived from different epidemiologic settings. Considerable genetic diversity and common antibiotic resistance were revealed among GGS strains which differed according to the epidemiologic settings. These results emphasize the importance of GGS infections and carriage. Further clinical, epidemiological and basic research of GGS as a pathogen is warranted.
References
Reissmann S, Friedrichs C, Rajkumari R et al (2010) Contribution of Streptococcus anginosus to infection caused by group C and G Streptococci, Southern India. Emerg Infect Dis 16:656–663
Asmah N, Eberspacher B, Regnath T, Arvand M (2009) Prevalence of erythromycin and clindamycin resistance among clinical isolates of the Streptococcus anginosus group in Germany. J Med Microbiol 58:222–227
Takahashi T, Ubukata K, Watanabe H (2011) Invasive infection caused by Streptococcus dysgalactiae subsp. equisimilis: characteristics of strains and clinical features. J Infect Chemother 17:1–10
Yin J, Yu S, Liu X et al (2012) Molecular characterization of group G Streptococcus dysgalactiae subsp. equisimilis recovered from patients and healthy people in China. Diagn Microbiol Infect Dis 72:41–46
Cohen-Poradosu R, Jaffe J, Lavi D et al (2004) Group G streptococcal bacteremia in Jerusalem. Emerg Infect Dis 10(8):1455–1460
Ahmad Y, Gertz RE Jr, Li Z et al (2009) Genetic Relationships deduced from emm and multilocus sequence typing of invasive Streptococcus dysgalactiae subsp. equisimilis and S. canis recovered from isolates collected in the United States. J Clin Microbiol 47:2046–2054
McDonald M, Towers RJ, Andrews RM, Carapetis JR, Currie BJ (2007) Epidemiology of Streptococcus dysgalactiae subsp. equisimilis in tropical communities, Northern Australia. Emerg Infect Dis 13:1694–1700
Rantala S, Vuopio VJ, Vuento R, Huhtala H, Syrjanen J (2009) Clinical presentations and epidemiology of beta haemolytic streptococcal bacteremia: a population based study. Clin Microbiol Infect 15:286–288
Cohen D, Ferne M, Rouach T, Bergner-Rabinowitz S (1987) Food-borne outbreak of group G streptococcal sore throat in an Israeli military base. Epidemiol Infect 99:249–55
Wasserzug O, Balicer RD, Boxman J, Klement E, Ambar R, Zimhony O (2011) A cluster of septic olecranon bursitis in association with infantry training. Mil Med 176:122–4
Broyles LN, Van Beneden C et al (2009) Population-based study of invasive disease due to beta-hemolytic streptococci of groups other than A and B. Clin Infect Dis 48:706–712
Levine H, Balicer RD, Zarka S et al (2010) Dynamics of Pneumococcal acquisition and carriage in young adults during training in confined settings in Israel. PLoS ONE 7:e46491
Bar A, Bar Zeev Y, Shleiom U, Burshtein S, Ankol O, Barenboim E (2009) A Foodborne Streptococcus G outbreak. J Military Med 6(4):146–8
Liu LC, Tsai JC, Hsueh PR, Teng LJ (2006) Rapid differentiation between members of the anginosus group and Streptococcus dysglactiae subsp. equisimilis within beta-hemolytic group C and G Streptococci by PCR. J Clin Microbiol 44:1836–1838
Abramson JH (2011) WINPEPI updated: computer programs for epidemiologists and their teaching potential. Epidemiol Perspect Innov 8:1
Menon T, Lloyd C, Malathy B, Sakota V, Jackson D, Beall B (2008) emm type diversity of beta-haemolytic streptococci recovered in Chennai, India. J Med Microbiol 57:540–2
Tseng SP, Lin YY, Tsai JC et al (2010) Distribution of emm types and genetic characterization of the mgc locus in group G Streptococcus dysgalactiae subsp. equisimilis from a hospital in Northern Taiwan. J Clin Microbiol 48:2975–2977
Behera B, Mathur P, Bhardwaj N, Jain N, Misra MC, Kapil A, Singh S (2014) Antibiotic susceptibilities, streptococcal pyrogenic exotoxin gene profiles among clinical isolates of group C or G Streptococcus dysgalactiae subsp. equisimilis & of group G S. anginosus group at a tertiary care center. Indian J Med Res 139:438–445
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article has not been published elsewhere (in part or in full), and is not under consideration by another journal or other publication.
Conflict of interest
All authors declare following the rules of good scientific practice and report no conflict of interest related to this manuscript.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Tami Halperin and Hagai Levine contributed equally to this work.
Rights and permissions
About this article
Cite this article
Halperin, T., Levine, H., Korenman, Z. et al. Molecular characterization and antibiotic resistance of group G streptococci in Israel: comparison of invasive, non-invasive and carriage isolates. Eur J Clin Microbiol Infect Dis 35, 1649–1654 (2016). https://doi.org/10.1007/s10096-016-2705-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2705-x