Abstract
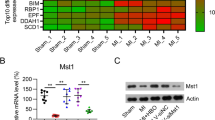
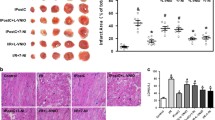
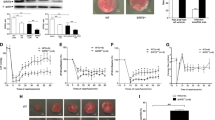
Several potential sources of reactive oxygen species (ROS) in cells exist. One source is NADPH oxidase, which is especially important for superoxide radical production. Nox2 is a primary regulatory subunit of NADPH oxidase. In the present study, we examined the role of ROS and NADPH oxidase in ischemic preconditioning (IP)-mediated cardioprotection by using Nox2−/− mice. Both wild-type (WT) and Nox2−/− mice were subjected to either 30 min of ischemia followed by 2 h of reperfusion (IR) or IP prior to 30 min ischemia and 2 h of reperfusion. Reduction in left ventricular developed pressure (60.1 versus 63 mmHg), dp/dt max (893 versus 1,027 mmHg/s), and aortic flow (0.9 versus 1.8 ml/min) was observed in Nox2−/−IPIR compared to WTIPIR along with increased infarct size (33% versus 22%) and apoptosis after 120 min of reperfusion. Differentially regulated genes were demonstrated by comparing gene expression in WTIPIR versus Nox2−/− IPIR hearts. Selected differentially regulated genes such as β-catenin, SRPK3, ERDR1, ACIN1, Syntaxin-8, and STC1 were validated by real-time PCR. Taken together, this is the first report identifying important, differentially expressed genes during ischemic preconditioning in Nox2−/− mice by using microarray analysis.






Similar content being viewed by others
References
Addya S, Shiroto K, Turoczi T, Zhan L, Kaga S, Fukuda S, Surrey S, Duan L, Fong G, Yamamoto F (2005) Ischemic preconditioning-mediated cardioprotection is disrupted in heterozygous Flt-1 (VEGFR-1) knockout mice. J Mol Cell Cardiol 38(2):345–351
Adluri RS, Thirunavukkarasu M, Dunna NR, Zhan L, Oriowo B, Takeda K, Sanchez J, Otani H, Maulik G, Fong GH, Maulik N (2010) Disruption of HIF-prolyl hydroxylase-1 (PHD-1−/−) attenuates ex vivo myocardial ischemia/reperfusion injury through HIF-1alpha transcription factor and its target genes in mice. Antioxid Redox Signal. 15(7):1789–1797
Anilkumar N, Sirker A, Shah A (2009) Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci J Virtual Library 14:3168–3187
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313
Bell R, Cave A, Johar S, Hearse D, Shah A, Shattock M (2005) Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. FASEB J 19:2037–2039
Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM (2006) NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8(5–6):691–728
Chen W, Gabel S, Steenbergen C, Murphy E (1995) A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res 77(2):424
Frantz S, Brandes RP, Hu K, Rammelt K, Wolf J, Scheuermann H, Ertl G, Bauersachs J (2006) Left ventricular remodeling after myocardial infarction in mice with targeted deletion of the NADPH oxidase subunit gp91PHOX. Basic Res Cardiol 101(2):127–132
Fukuda S, Kaga S, Sasaki H, Zhan L, Zhu L, Otani H, Kalfin R, Das DK, Maulik N (2004) Angiogenic signal triggered by ischemic stress induces myocardial repair in rat during chronic infarction. J Mol Cell Cardiol 36(4):547–559
Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM (2002) Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc Natl Acad Sci U S A 99(3):1182–1187
Giordano F (2005) Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115(3):500–508
Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E (2003) Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol 161(4):793–804
Hausenloy D, Yellon D (2008) Preconditioning and postconditioning: new strategies for cardioprotection. Diabetes Obes Metab 10(6):451–459
Hayashi T, Yamashita C, Matsumoto C, Kwak CJ, Fujii K, Hirata T, Miyamura M, Mori T, Ukimura A, Okada Y, Matsumura Y, Kitaura Y (2008) Role of gp91phox-containing NADPH oxidase in left ventricular remodeling induced by intermittent hypoxic stress. Am J Physiol Heart Circ Physiol 294(5):H2197–H2203
Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kyto V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R (2010) Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem 285(32):24487–24493
La Porta C (2010) AQP1 is not only a water channel: It contributes to cell migration through Lin7/beta-catenin. Cell Adh Migr 4(2):204–206
Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4(3):181–189
Lassegue B, Clempus R (2003) Vascular NAD (P) H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285(2):277
Li J, Zuan W, Yan R, Tropak MB, Jean-st-Michel J, Liang W, Gladstone R, Backx PH, Kharbanda RK and Redington AN (2011) Remote preconditioning provides potent cardioprotection via PI3K/Akt activation and is associated with nuclear accumulation of b-catenin. Clin Sci (Lond) 120:451–462
Maulik N (2004) Ischemic preconditioning mediated angiogenic response in the heart. Antioxid Redox Signal 6(2):413–421
Mohazzab-H K, Kaminski P, Wolin M (1997) Lactate and PO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation 96(2):614
Moncho-Amor V, Ibanez de Caceres I, Bandres E, Martinez-Poveda B, Orgaz JL, Sanchez-Perez I, Zazo S, Rovira A, Albanell J, Jimenez B, Rojo F, Belda-Iniesta C, Garcia-Foncillas J, Perona R (2011) DUSP1/MKP1 promotes angiogenesis, invasion and metastasis in non-small-cell lung cancer. Oncogene 30(6):668–678
Murry C, Richard V, Jennings R, Reimer K (1988) Preconditioning with ischemia: is the protective effect mediated by free radical-induced myocardial stunning. Circulation 78(Suppl II):77
Nakagami H, Jensen KS, Liao JK (2003) A novel pleiotropic effect of statins: prevention of cardiac hypertrophy by cholesterol-independent mechanisms. Ann Med 35(6):398–403
Nakagawa O, Arnold M, Nakagawa M, Hamada H, Shelton JM, Kusano H, Harris TM, Childs G, Campbell KP, Richardson JA, Nishino I, Olson EN (2005) Centronuclear myopathy in mice lacking a novel muscle-specific protein kinase transcriptionally regulated by MEF2. Genes Dev 19(17):2066–2077
Nakano A, Cohen M, Downey J (2000) Ischemic preconditioning: from basic mechanisms to clinical applications. Pharmacol Ther 86(3):263–275
Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D (2002) VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J 21(18):4885–4895
Otani H (2008) Ischemic preconditioning: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 10(2):207–248
Penna C, Mancardi D, Rastaldo R, Pagliaro P (2009) Cardioprotection: a radical view Free radicals in pre and postconditioning. Biochim Biophys Acta 1787(7):781–793
Peralta C, Bulbena O, Xaus C, Prats N, Cutrin J, Poli G, Gelpi E, Rosello-Catafau J (2002) Ischemic preconditioning: a defense mechanism against the reactive oxygen species generated after hepatic ischemia reperfusion1. Transplantation 73(8):1203
Petry A, Weitnauer M, Gorlach A (2010) Receptor activation of NADPH oxidases. Antioxid Redox Signal 13(4):467–487
Rada B, Leto TL (2008) Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol 15:164–187
Ran X, Wang H, Chen Y, Zeng Z, Zhou Q, Zheng R, Sun J, Wang B, Lv X, Liang Y, Zhang K, Liu W (2010) Aquaporin-1 expression and angiogenesis in rabbit chronic myocardial ischemia is decreased by acetazolamide. Hear Vessel 25(3):237–247
Rhee S (1999) Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 31(2):53
Sako K, Fukuhara S, Minami T, Hamakubo T, Song H, Kodama T, Fukamizu A, Gutkind JS, Koh GY, Mochizuki N (2009) Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J Biol Chem 284(9):5592–5601
Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K (2005) Glycogen-synthase kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res 96(3):308–318
Thirunavukkarasu M, Addya S, Juhasz B, Pant R, Zhan L, Surrey S, Maulik G, Menon V, Maulik N (2008a) Heterozygous disruption of Flk-1 receptor leads to myocardial ischaemia reperfusion injury in mice: application of affymetrix gene chip analysis. J Cell Mol Med 12(4):1284–1302
Thirunavukkarasu M, Han Z, Zhan L, Penumathsa SV, Menon VP, Maulik N (2008b) Adeno-sh-beta-catenin abolishes ischemic preconditioning-mediated cardioprotection by downregulation of its target genes VEGF, Bcl-2, and survivin in ischemic rat myocardium. Antioxid Redox Signal 10(8):1475–1484
Van Gelder R, von Zastrow M, Yool A, Dement W, Barchas J, Eberwine J (1990) Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci U S A 87(5):1663
Vanden Hoek T, Becker L, Shao Z, Li C, Schumacker P (1998) Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273(29):18092
Wang Y, Huang L, Abdelrahim M, Cai Q, Truong A, Bick R, Poindexter B, Sheikh-Hamad D (2009) Stanniocalcin-1 suppresses superoxide generation in macrophages through induction of mitochondrial UCP2. J Leukoc Biol 86(4):981–988
Westberg JA, Serlachius M, Lankila P, Andersson LC (2007) Hypoxic preconditioning induces elevated expression of stanniocalcin-1 in the heart. Am J Physiol Heart Circ Physiol 293(3):H1766–H1771
Wu J, Bohanan CS, Neumann JC, Lingrel JB (2008) KLF2 transcription factor modulates blood vessel maturation through smooth muscle cell migration. J Biol Chem 283(7):3942–3950
Zhang K, Lindsberg PJ, Tatlisumak T, Kaste M, Olsen HS, Andersson LC (2000) Stanniocalcin: a molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci U S A 97(7):3637–3642
Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM (2010) NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A 107(42):18121–18126
Acknowledgments
This study was supported by the National Institute of Health (USA) grants HL-56803, HL-69910, and HL-85804 to NM.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Table S1
Top 5 gene networks with high scores (>10) in WTIR versus Nox2−/− IR comparison. (DOC 29 kb)
Table S2
Top 5 gene networks with high scores (>10) in WTIPIR versus Nox2−/− IPIR comparison. (DOC 29 kb)
Table S3
Functional analysis of the differentially expressed genes identified in WTIR versus Nox2−/− IR comparison. (DOC 94 kb)
Table S4
Functional analysis of the differentially expressed genes identified in WTIPIR versus Nox2−/− IPIR comparison. (DOC 115 kb)
Table S5
Pathway analysis of the genes affected in WTIR versus Nox2−/− IR comparison. (DOC 122 kb)
Table S6
Pathway analysis of the genes affected in WTIPIR versus Nox2−/− IPIR comparison. (DOC 126 kb)
Rights and permissions
About this article
Cite this article
Thirunavukkarasu, M., Adluri, R.S., Juhasz, B. et al. Novel role of NADPH oxidase in ischemic myocardium: a study with Nox2 knockout mice. Funct Integr Genomics 12, 501–514 (2012). https://doi.org/10.1007/s10142-011-0256-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-011-0256-x