Abstract
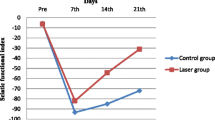
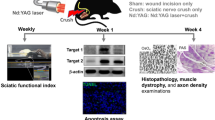
The objective of the study was to investigate the efficacy of three energy densities 4, 10, and 50 J/cm2 of pulsed Nd:YAG laser for the treatment of crushed sciatic nerve in Wister rats by evaluating changes in the sciatic functional index and the electrophysiology.A total of 180 Wistar rats were involved in the study. Rats were randomly assigned to five groups. Rats were subjected to the sciatic nerve crushing. Control negative (CONT-ve), which received no crushing; control positive (CONT+ve), which received crushing with no laser; and HILT-4, HILT-10, and HILT-50 groups, which received pulsed Nd:YAG laser (10 Hz, 360 mJ/cm2) with energy densities 4, 10, and 50 J/cm2, respectively. The SFI, the amilitude of compound motor action potential (CMAP) and sciatic motor nerve conduction velocity (MNCV) were measured before and after seven, 14, and 21 days after crushing. For the SFI and electrophysiological analysis, repeated measures ANOVA is used, followed by Bonferroni’s repeated-measures test. Statistical significance was set at p < 0.05. After one week, there was no significant difference in SFI, CMAP, and MNCV among the three laser groups with significant changes between them and CONT-ve and CONT+ve groups. There was a significant increase in either CMAP amplitude or MNCV after 14 days with significant decrease in the SFI after 21 days among all treatment groups. The pulsed Nd:YAG laser applied with energy densities 4, 10, and 50 J/cm2 significantly decreased the SFI and increased the CMAP and MNCV of the crushed sciatic nerve in Wister rats. Among laser doses, the difference in the rate of recovery in the electrophysiology was found after two weeks while in the SFI after three weeks. The improvement after the nerve injury was time and dose dependent.




Similar content being viewed by others
References
de Souza LG, Marcolino AM, Kuriki HU, Goncalves ECD, Fonseca MCR, Barbosa RI (2018) Comparative effect of photobiomodulation associated with dexamethasone after sciatic nerve injury model. Lasers Med Sci 33(6):1341–1349. https://doi.org/10.1007/s10103-018-2494-9
Anders JJ, Moges H, Wu X, Erbele ID, Alberico SL, Saidu EK, Smith JT, Pryor BA (2014) In vitro and in vivo optimization of infrared laser treatment for injured peripheral nerves. Lasers Surg Med 46(1):34–45. https://doi.org/10.1002/lsm.22212
Andraus RAC, Maia LP, de Souza Lino AD, Fernandes KBP, de Matos Gomes MV, de Jesus Guirro RR, Barbieri CH (2017) LLLT actives MMP-2 and increases muscle mechanical resistance after nerve sciatic rat regeneration. Lasers Med Sci 32(4):771–778. https://doi.org/10.1007/s10103-017-2169-y
Wang CZ, Chen YJ, Wang YH, Yeh ML, Huang MH, Ho ML, Liang JI, Chen CH (2014) Low-level laser irradiation improves functional recovery and nerve regeneration in sciatic nerve crush rat injury model. PLoS One 9(8):e103348. https://doi.org/10.1371/journal.pone.0103348
Navarro X, Vivo M, Valero-Cabre A (2007) Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82(4):163–201. https://doi.org/10.1016/j.pneurobio.2007.06.005
Hoffman PN (2010) A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp Neurol 223(1):11–18. https://doi.org/10.1016/j.expneurol.2009.09.006
Yarar E, Kuruoglu E, Kocabicak E, Altun A, Genc E, Ozyurek H, Kefeli M, Marangoz AH, Aydin K, Cokluk C (2015) Electrophysiological and histopathological effects of mesenchymal stem cells in treatment of experimental rat model of sciatic nerve injury. Int J Clin Exp Med 8(6):8776–8784
Gigo-Benato D, Russo TL, Tanaka EH, Assis L, Salvini TF, Parizotto NA (2010) Effects of 660 and 780 nm low-level laser therapy on neuromuscular recovery after crush injury in rat sciatic nerve. Lasers Surg Med 42(9):673–682. https://doi.org/10.1002/lsm.20978
Barbosa RI, Marcolino AM, de Jesus Guirro RR, Mazzer N, Barbieri CH, de Cassia Registro Fonseca M (2010) Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci 25(3):423–430. https://doi.org/10.1007/s10103-009-0750-8
Marcolino AM, Barbosa RI, das Neves LM, Mazzer N, de Jesus Guirro RR, de Cassia Registro Fonseca M (2013) Assessment of functional recovery of sciatic nerve in rats submitted to low-level laser therapy with different fluences. An experimental study: laser in functional recovery in rats. J Hand Microsurg 5(2):49–53. https://doi.org/10.1007/s12593-013-0096-0
Andreo L, Soldera CB, Ribeiro BG, de Matos PRV, Bussadori SK, Fernandes KPS, Mesquita-Ferrari RA (2017) Effects of photobiomodulation on experimental models of peripheral nerve injury. Lasers Med Sci 32(9):2155–2165. https://doi.org/10.1007/s10103-017-2359-7
de Oliveira RF, de Andrade Salgado DM, Trevelin LT, Lopes RM, da Cunha SR, Aranha AC, de Paula EC, de Freitas PM (2015) Benefits of laser phototherapy on nerve repair. Lasers Med Sci 30(4):1395–1406. https://doi.org/10.1007/s10103-014-1531-6
Takhtfooladi MA, Jahanbakhsh F, Takhtfooladi HA, Yousefi K, Allahverdi A (2015) Effect of low-level laser therapy (685 nm, 3 J/cm(2)) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 30(3):1047–1052. https://doi.org/10.1007/s10103-015-1709-6
Ziago EK, Fazan VP, Iyomasa MM, Sousa LG, Yamauchi PY, da Silva EA, Borie E, Fuentes R, Dias FJ (2017) Analysis of the variation in low-level laser energy density on the crushed sciatic nerves of rats: a morphological, quantitative, and morphometric study. Lasers Med Sci 32(2):369–378. https://doi.org/10.1007/s10103-016-2126-1
Akgul T, Gulsoy M, Gulcur HO (2014) Effects of early and delayed laser application on nerve regeneration. Lasers Med Sci 29(1):351–357. https://doi.org/10.1007/s10103-013-1355-9
Silva-Couto MA, Gigo-Benato D, Tim CR, Parizotto NA, Salvini TF, Russo TL (2012) Effects of low-level laser therapy after nerve reconstruction in rat denervated soleus muscle adaptation. Revista brasileira de fisioterapia (Sao Carlos (Sao Paulo, Brazil)) 16(4):320–327
Sene GA, Sousa FF, Fazan VS, Barbieri CH (2013) Effects of laser therapy in peripheral nerve regeneration. Acta Ortopedica Brasileira 21(5):266–270. https://doi.org/10.1590/s1413-78522013000500005
Sousa FF, Ribeiro TL, Fazan VP, Barbieri CH (2013) Lack of effectiveness of laser therapy applied to the nerve course and the correspondent medullary roots. Acta ortopedica brasileira 21(2):92–97. https://doi.org/10.1590/s1413-78522013000200005
Huang YY, Chen AC, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose-response : a publication of International Hormesis Society 7(4):358–383. https://doi.org/10.2203/dose-response.09-027.Hamblin
Huang YY, Sharma SK, Carroll J, Hamblin MR (2011) Biphasic dose response in low level light therapy - an update. Dose-response : a publication of International Hormesis Society 9(4):602–618. https://doi.org/10.2203/dose-response.11-009.Hamblin
Kheshie AR, Alayat MS, Ali MM (2014) High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: a randomized controlled trial. Lasers Med Sci 29(4):1371–1376. https://doi.org/10.1007/s10103-014-1529-0
Alayat MSM, Aly THA, Elsayed AEM, Fadil ASM (2017) Efficacy of pulsed Nd: YAG laser in the treatment of patients with knee osteoarthritis: a randomized controlled trial. Lasers Med Sci 32(3):503–511
Alayat MSM, Atya AM, Ali MME, Shosha TM (2014) Long-term effect of high-intensity laser therapy in the treatment of patients with chronic low back pain: a randomized blinded placebo-controlled trial. Lasers Med Sci 29(3):1065–1073
Alayat MSM, Alshehri MA, Shousha TM, Abdelgalil AA, Al-Attar WS, Alhasan H, Khayyat OK (2019) The effectiveness of high intensity laser therapy in the management of spinal disorders: a systematic review and meta-analysis. J Back musculoskelet Rehabil. https://doi.org/10.3233/bmr-181341
Alayat MS, Mohamed AA, Helal OFE, Khaled OA (2016) Efficacy of high-intensity laser therapy in the treatment of chronic neck pain: a randomized double-blind placebo-control trial. Lasers Med Sci 31(4):687–694. https://doi.org/10.1007/s10103-016-1910-2
Dundar U, Turkmen U, Toktas H, Solak O, Ulasli AM (2015) Effect of high-intensity laser therapy in the management of myofascial pain syndrome of the trapezius: a double-blind, placebo-controlled study. Lasers Med Sci 30(1):325–332. https://doi.org/10.1007/s10103-014-1671-8
Elsodany AM, Alayat MSM, Ali MME, Khaprani HM (2018) Long-term effect of pulsed Nd:YAG laser in the treatment of patients with rotator cuff tendinopathy: a randomized controlled trial. Photomed Laser Surg 36(9):506–513. https://doi.org/10.1089/pho.2018.4476
Alayat MSM, Elsodany AM, El Fiky AAR (2014) Efficacy of high and low level laser therapy in the treatment of Bell's palsy: a randomized double blind placebo-controlled trial. Lasers Med Sci 29(1):335–342
de Medinaceli L, DeRenzo E, Wyatt RJ (1984) Rat sciatic functional index data management system with digitized input. Comp Biomed Res Int J 17(2):185–192
Bain JR, Mackinnon SE, Hunter DA (1989) Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83(1):129–138
Campbell WW (2008) Evaluation and management of peripheral nerve injury. Clin Neurophysiol : official journal of the International Federation of Clinical Neurophysiology 119(9):1951–1965. https://doi.org/10.1016/j.clinph.2008.03.018
Geuna S (2015) The sciatic nerve injury model in pre-clinical research. J Neurosci Methods 243:39–46. https://doi.org/10.1016/j.jneumeth.2015.01.021
de Almeida Melo Maciel Mangueira M, Maciel Mangueira N, Pereira Gama Filho O, Moyses de Oliveira M, Albuquerque Heluy R, Silveira L Jr, Caparelli Moniz de Aragao Daquer E (2019) Biochemical changes in injured sciatic nerve of rats after low-level laser therapy (660 nm and 808 nm) evaluated by Raman spectroscopy. Lasers Med Sci 34(3):525–535. https://doi.org/10.1007/s10103-018-2627-1
de Almeida Melo Maciel Mangueira M, Maciel Mangueira N, Pereira Gama Filho O, Moyses de Oliveira M, Albuquerque Heluy R, Silveira L Jr, Caparelli Moniz de Aragao Daquer E (2018) Biochemical changes in injured sciatic nerve of rats after low-level laser therapy (660 nm and 808 nm) evaluated by Raman spectroscopy. Lasers Med Sci. https://doi.org/10.1007/s10103-018-2627-1
Reis F, Belchior A, Nicolau R, Fonseca T, Carvalho P (2008) Efeito da terapia com laser de arsenieto de gálio e alumínio (660Nm) sobre a recuperação do nervo ciático de ratos após lesão por neurotmese seguida de anastomose epineural: análise funcional. Braz J Physical Ther 12:215–221
de Medinaceli L, Freed WJ, Wyatt RJ (1982) An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 77(3):634–643
Bae CS, Lim SC, Kim KY, Song CH, Pak S, Kim SG, Jang CH (2004) Effect of Ga-as laser on the regeneration of injured sciatic nerves in the rat. In vivo (Athens, Greece) 18(4):489–495
Song HJ, Seo HJ, Lee Y, Kim SK (2018) Effectiveness of high-intensity laser therapy in the treatment of musculoskeletal disorders: a systematic review and meta-analysis of randomized controlled trials. Medicine 97(51):e13126. https://doi.org/10.1097/md.0000000000013126
Serafim KG, Ramos Sde P, de Lima FM, Carandina M, Ferrari O, Dias IF, Toginho Filho Dde O, Siqueira CP (2012) Effects of 940 nm light-emitting diode (led) on sciatic nerve regeneration in rats. Lasers Med Sci 27(1):113–119. https://doi.org/10.1007/s10103-011-0923-0
Medalha CC, Di Gangi GC, Barbosa CB, Fernandes M, Aguiar O, Faloppa F, Leite VM, Renno AC (2012) Low-level laser therapy improves repair following complete resection of the sciatic nerve in rats. Lasers Med Sci 27(3):629–635. https://doi.org/10.1007/s10103-011-1008-9
Oliveira FB, Pereira VM, da Trindade AP, Shimano AC, Gabriel RE, Borges AP (2012) Action of therapeutic laser and ultrasound in peripheral nerve regeneration. Acta ortopedica brasileira 20(2):98–103. https://doi.org/10.1590/s1413-78522012000200008
Khullar SM, Brodin P, Messelt EB, Haanaes HR (1995) The effects of low level laser treatment on recovery of nerve conduction and motor function after compression injury in the rat sciatic nerve. Eur J Oral Sci 103(5):299–305
dos Reis FA, Belchior AC, de Carvalho PT, da Silva BA, Pereira DM, Silva IS, Nicolau RA (2009) Effect of laser therapy (660 nm) on recovery of the sciatic nerve in rats after injury through neurotmesis followed by epineural anastomosis. Lasers Med Sci 24(5):741–747. https://doi.org/10.1007/s10103-008-0634-3
Zhang LX, Tong XJ, Yuan XH, Sun XH, Jia H (2010) Effects of 660-nm gallium-aluminum-arsenide low-energy laser on nerve regeneration after acellular nerve allograft in rats. Synapse (New York, NY) 64(2):152–160. https://doi.org/10.1002/syn.20724
Shen CC, Yang YC, Liu BS (2011) Large-area irradiated low-level laser effect in a biodegradable nerve guide conduit on neural regeneration of peripheral nerve injury in rats. Injury 42(8):803–813. https://doi.org/10.1016/j.injury.2011.02.005
Shen CC, Yang YC, Huang TB, Chan SC, Liu BS (2013) Neural regeneration in a novel nerve conduit across a large gap of the transected sciatic nerve in rats with low-level laser phototherapy. J Biomed Mater Res A 101(10):2763–2777. https://doi.org/10.1002/jbm.a.34581
Bagis S, Comelekoglu U, Coskun B, Milcan A, Buyukakilli B, Sahin G, Ozisik S, Erdogan C (2003) No effect of GA-AS (904 nm) laser irradiation on the intact skin of the injured rat sciatic nerve. Lasers Med Sci 18(2):83–88. https://doi.org/10.1007/s10103-003-0258-6
Diker N, Aytac D, Helvacioglu F, Oguz Y (2019) Comparative effects of photobiomodulation therapy at wavelengths of 660 and 808 nm on regeneration of inferior alveolar nerve in rats following crush injury. Lasers Med Sci. https://doi.org/10.1007/s10103-019-02838-w
Ash C, Dubec M, Donne K, Bashford T (2017) Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med Sci 32(8):1909–1918. https://doi.org/10.1007/s10103-017-2317-4
Ansari MA, Mohajerani E (2011) Mechanisms of laser-tissue interaction: I Optical Properties of Tissue. J Lasers Med Sci 2(3):119–125
Gigo-Benato D, Geuna S, Rochkind S (2005) Phototherapy for enhancing peripheral nerve repair: a review of the literature. Muscle Nerve 31(6):694–701. https://doi.org/10.1002/mus.20305
Alcantara CC, Gigo-Benato D, Salvini TF, Oliveira AL, Anders JJ, Russo TL (2013) Effect of low-level laser therapy (LLLT) on acute neural recovery and inflammation-related gene expression after crush injury in rat sciatic nerve. Lasers Surg Med 45(4):246–252. https://doi.org/10.1002/lsm.22129
Song JW, Li K, Liang ZW, Dai C, Shen XF, Gong YZ, Wang S, Hu XY, Wang Z (2017) Low-level laser facilitates alternatively activated macrophage/microglia polarization and promotes functional recovery after crush spinal cord injury in rats. Sci Rep 7(1):620. https://doi.org/10.1038/s41598-017-00553-6
de Andrade ALM, Bossini PS, do Canto De Souza ALM, Sanchez AD, Parizotto NA (2017) Effect of photobiomodulation therapy (808 nm) in the control of neuropathic pain in mice. Lasers Med Sci 32(4):865–872. https://doi.org/10.1007/s10103-017-2186-x
Rocha IR, Ciena AP, Rosa AS, Martins DO, Chacur M (2017) Photobiostimulation reverses allodynia and peripheral nerve damage in streptozotocin-induced type 1 diabetes. Lasers Med Sci 32(3):495–501. https://doi.org/10.1007/s10103-016-2140-3
Amaroli A, Ravera S, Baldini F, Benedicenti S, Panfoli I, Vergani L (2019) Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med Sci 34(3):495–504. https://doi.org/10.1007/s10103-018-2623-5
Funding
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology - the Kingdom of Saudi Arabia, award number (12-MED 2958-10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Research Ethical Committee, Faculty of Applied Medical Science, Umm Al-Qura University, approved the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alayat, M.S.M., Basalamah, M.A., Elbarrany, W.G.E.AE. et al. Dose-dependent effect of the pulsed Nd:YAG laser in the treatment of crushed sciatic nerve in Wister rats: an experimental model. Lasers Med Sci 35, 1989–1998 (2020). https://doi.org/10.1007/s10103-020-02999-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-02999-z