Abstract
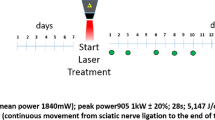
The aim of this study was to identify biochemical changes in sciatic nerve (SN) after crush injury and low-level laser therapy (LLLT) with 660 nm and 808 nm by Raman spectroscopy (RS) analysis. A number of 32 Wistar rats were used, divided into four groups (control 1, control 2, LASER 660 nm, and LASER 808 nm). All animals underwent surgical procedure of the SN and groups control 2, LASER 660 nm, and LASER 808 nm were submitted to SN crush damage (axonotmesis). The LLLT in the groups LASER 660 nm and LASER 808 nm was applied daily for 21 consecutive days (100 mW, 30 s, 133 J/cm2 fluence). The hind paw was removed and the SN was dissected and positioned on an aluminum support to collect dispersive Raman spectra (830 nm excitation, 30 s accumulation). To estimate the biochemical changes in the SN associated with LLLT, the principal component analysis (PCA) was applied. The Raman spectra of the sciatic nerve fragments showed peaks of the major biochemical components of the nerve, especially sphingolipids, phospholipids, glycoproteins, and collagen. The spectral features identified in some of the principal component loading vectors are referred to the biochemical elements present on the SN and were increased in the groups treated with LLLT, mainly lipids (sphingo and phospholipids) and proteins (collagen)—constituents of the myelin sheath. The RS was effective in identifying the biochemical differences in the SN after the crush injury, and LASER 660 nm was more efficient than the LASER 808 nm in cell proliferation and repair of the injured SN.





Similar content being viewed by others
References
Hoffman PN (2010) A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp Neurol 223(1):11–18. https://doi.org/10.1016/j.expneurol.2009.09.006
Seddon HJ (1947) The use of autogenous grafts for the repair of large gaps in peripheral nerves. Br J Surg 35(138):151–167. https://doi.org/10.1002/bjs.18003513808
Sunderland S (1951) The function of nerve fibers whose structure has been disorganized. Anat Rec 109(3):503–513. https://doi.org/10.1002/ar.1091090307
Ciaramitaro P, Mondelli M, Logullo F, Grimaldi S, Battiston B, Sard A, Scarinzi C, Migliaretti G, Faccani G, Cocito D (2010) Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst 15(2):120–127. https://doi.org/10.1111/j.1529-8027.2010.00260.x
Wu SG, Huang SJ, Zhou J, Sun JY, Guo H, Li FY et al (2014) Dosimetric analysis of the brachial plexus among patients with breast cancer treated with post-mastectomy radiotherapy to the ipsilateral supraclavicular area: report of 3 cases of radiation-induced brachial plexus neuropathy. Radiat Oncol 9(1):1–7. https://doi.org/10.1186/s13014-014-0292-5
Brull R, Hadzic A, Reina MA, Barrington MJ (2015) Pathophysiology and etiology of nerve injury following peripheral nerve blockade. Reg Anesth Pain Med 40(5):479–490. https://doi.org/10.1097/AAP.0000000000000125
Pereira S, Fontes F, Sonin T, Dias T, Fragoso M, Castro-Lopes JM, Lunet N (2016) Chemotherapy-induced peripheral neuropathy after neoadjuvant or adjuvant treatment of breast cancer: a prospective cohort study. Support Care Cancer 24(4):1571–1581. https://doi.org/10.1007/s00520-015-2935-y
Madl CM, Heilshorn SC (2015) Matrix interactions modulate neurotrophinmediated neurite outgrowth and pathfinding. Neural Regen Res 10(4):514–517. https://doi.org/10.4103/1673-5374.155426
Elbaz AA, Abu-Almaaty AH, Hassan MK, Mohammed EA, Aziz MM (2017) Therapeutic role of Schwann cells and resveratrol or melatonin combination in peripheral nerve injured rat models. J Exp Appl Anim Sci 2(2):190–210. https://doi.org/10.20454/jeaas.2017.1285
Terengui G, Hart A, Wiberg M (2011) The nerve injury and the dying neurons: diagnosis and prevention. J Hand Surg Eur Vol 36(9):730–734. https://doi.org/10.1177/1753193411422202
Campbell WW (2008) Evaluation and management of peripheral nerve injury. Clin Neurophysiol 119(9):1951–1965. https://doi.org/10.1016/j.clinph.2008.03.018
Geuna S (2015) The sciatic nerve injury model in pre-clinical research. J Neurosci Methods 30(243):39–46. https://doi.org/10.1016/j.jneumeth.2015.01.021
Morisaki S, Ota C, Matsuda K, Kaku N, Fujiwara H, Oda R et al (2013) Application of Raman spectroscopy for visualizing biochemical changes during peripheral nerve injury in vitro and in vivo. J Biomed Opt 18(11):116011–116018. https://doi.org/10.1117/1.JBO.18.11.116011
Hanlon EB, Manoharan R, Koo TW, Shafer KE, Motz JT, Fitzmaurice M et al (2000) Prospects for in vivo Raman spectroscopy. Phys Med Biol 45(2):R1–R59. https://doi.org/10.1088/0031-9155/45/2/201
Moura Júnior MJ, Maia Filho AL, Pessoa DR, Alves MD, Justino Jde S, Andrade Mdos S et al (2015) Assessing the biochemical changes of tendons of rats in an experimental model of tenotomy under therapeutic ultrasound and leds (625 and 945 nm) by near-infrared Raman spectroscopy. Lasers Med Sci 30(6):1729–1738. https://doi.org/10.1007/s10103-015-1779-5
Mangueira NM, Xavier M, de Souza RA, Salgado MAC, Silveira L Jr, Villaverde AB (2015) Effect of low-level laser therapy in an experimental model of osteoarthritis in rats evaluated through Raman spectroscopy. J Photomed Laser Surg 33(3):145–153. https://doi.org/10.1089/pho.2014.3744
de Souza RA, Xavier M, Mangueira NM, Santos AP, Pinheiro AL, Villaverde AB, LJr S (2014) Raman spectroscopy detection of molecular changes associated with two experimental models of osteoarthritis in rats. Lasers Med Sci 29(2):797–804. https://doi.org/10.1007/s10103-013-1423-1
de Oliveira RF, de Andrade Salgado DM, Trevelin LT, Lopes RM, da Cunha SR, Aranha AC et al (2015) Benefits of laser phototherapy on nerve repair. Lasers Med Sci 30(4):1395–1406. https://doi.org/10.1007/s10103-014-1531-6
Andreo L, Soldera CB, Ribeiro BG, de Matos PRV, Bussadori SK, Fernandes KPS, Mesquita-Ferrari RA (2017) Effects of photobiomodulation on experimental models of peripheral nerve injury. Lasers Med Sci 32(9):2155–2165. https://doi.org/10.1007/s10103-017-2359-7
Andraus RAC, Maia LP, de Souza Lino AD, Fernandes KBP, de Matos Gomes MV et al (2017) LLLT actives MMP-2 and increases muscle mechanical resistance after nerve sciatic rat regeneration. Lasers Med Sci 32(4):771–778. https://doi.org/10.1007/s10103-017-2169-y
Takhtfooladi MA, Jahanbakhsh F, Takhtfooladi HA, Yousefi K, Allahverdi A (2015) Effect of low-level laser therapy (685 nm, 3 J/cm2) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 30(3):1047–1052. https://doi.org/10.1007/s10103-015-1709-6
Ziago EKM, Fazan VPS, Iyomasa MM, Sousa LG, Yamauchi PY, da Silva EA et al (2017) Analysis of the variation in low-level laser energy density on the crushed sciatic nerves of rats: a morphological, quantitative, and morphometric study. Lasers Med Sci 32(2):369–378. https://doi.org/10.1007/s10103-016-2126-1
Seabra ID, Pompeu E, Valenti MLG. Anestesia e analgesia de animais utilizados em protocolos experimentais. Central Vivarium of the University of São Paulo Medical School http://www.bioterio.fm.usp.br/pdf/Anestesia_e_Analgesia.pdf. Accessed 08 Mar 2018
Handwerker HO, Kobal G (1993) Psychophysiology of experimentally induced pain. Physiol Rev 73(3):639–671. https://doi.org/10.1152/physrev.1993.73.3.639
Bispo JA, Vieira EES, Silveira L, Fernandes AB (2013) Correlating the amount of urea, creatinine, and glucose in urine from patients with diabetes mellitus and hypertension with the risk of developing renal lesions by means of Raman spectroscopy and principal component analysis. J Biomed Opt 18(8):87004–870011. https://doi.org/10.1117/1.JBO.18.8.087004
Silveira L, Silveira FL, Bodanese B, Zângaro RA, Pacheco MT (2012) Discriminating model for diagnosis of basal cell carcinoma and melanoma in vitro based on the Raman spectra of selected biochemicals. J Biomed Opt 17(7):077003–077015. Erratum in: J Biomed Opt 18(3):039801. https://doi.org/10.1117/1.JBO.17.7.077003
Silveira L Jr, Borges RCF, Navarro RS, Giana HE, Zângaro RA, Pacheco MTT, Fernandes AB (2017) Quantifying glucose and lipid components in human serum by Raman spectroscopy and multivariate statistics. Lasers Med Sci 32(4):787–795. https://doi.org/10.1007/s10103-017-2173-2
Movasaghi Z, Rehman S, Rehman IU (2007) Raman spectroscopy of biological tissues. Appl Spectrosc 42(5):493–541. https://doi.org/10.1080/05704920701551530
Saher G, Stumpf SK (2015) Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim Biophys Acta 1851(8):1083–1094. https://doi.org/10.1016/j.bbalip.2015.02.010
Minamikawa T, Harada Y, Takamatsu T (2015) Ex vivo peripheral nerve detection of rats by spontaneous Raman spectroscopy. Sci Rep 5:17165–17176. https://doi.org/10.1038/srep17165
Krafft C, Neudert L, Simat T, Salzer R (2005) Near infrared Raman spectra of human brain lipids. Spectrochim Acta A Mol Biomol Spectrosc 61(7):1529–1535. https://doi.org/10.1016/j.saa.2004.11.017
Shetty G, Kedall C, Shepherd N, Stone N, Barr H (2006) Raman spectroscopy: evaluation of biochemical changes in carcinogenesis of oesophagus. Br J Cancer 94(10):1460–1464. https://doi.org/10.1038/sj.bjc.6603102
Stone N, Kendall C, Smith J, Crow P, Barr H (2004) Raman spectroscopy for identification of epithelial cancers. Faraday Discuss 126:141–157. https://doi.org/10.1039/B304992B
Cheng WT, Liu MT, Liu HN, Lin SY (2005) Micro-Raman spectroscopy used to identify and grade human skin pilomatrixoma. Microsc Res Tech 68(2):75–84. https://doi.org/10.1002/jemt.20229
Notingher I, Green C, Dyer C (2004) Discrimination between ricin and sulphur mustard toxicity in vitro using Raman spectroscopy. J R Soc Interface 1(1):79–90. https://doi.org/10.1098/rsif.2004.0008
Kateinen E, Elomaa M, Laakkonen UM, Sippola E, Niemela P, Suhonen J, Jarninen K (2007) Quatification of the amphetamine content in seized street samples by Raman spectroscopy. J Forensic Sci 52(1):88–92. https://doi.org/10.1111/j.1556-4029.2006.00306.x
Baron W, Hoekstra D (2010) On the biogenesis of myelin membranes: sorting, trafficking and cell polarity. FEBS Lett 584(9):1760–1770. https://doi.org/10.1016/j.febslet.2009.10.085
Schmitt S, Castelvetri LC, Simons M (2015) Metabolism and functions of lipids in myelin. Biochim Biophys Acta 1851(8):999–1005. https://doi.org/10.1016/j.bbalip.2014.12.016
Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH Jr, Murphy RC et al (2005) A comprehensive classification system for lipids. J Lipid Res 46(5):839–861. https://doi.org/10.1194/jlr.E400004-JLR200
Zöller I, Meixner M, Hartmann D, Büssow H, Meyer R, Gieselmann V, Eckhardt M (2008) Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J Neurosci 28(39):9741–9754. https://doi.org/10.1523/JNEUROSCI.0458-08.2008
Lahiri S, Futerman AH (2007) The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci 64(17):2270–2284. https://doi.org/10.1007/s00018-007-7076-0
Aguiar RP, Silveira L Jr, Falcão ET, Pacheco MT, Zângaro RA, Pasqualucci CA (2013) Discriminating neoplastic and normal brain tissues in vitro through Raman spectroscopy: a principal components analysis classification model. Photomed Laser Surg 31(12):595–604. https://doi.org/10.1089/pho.2012.3460
Barbosa RI, Marcolino AM, de Jesus GRR, Barbieri CH, de Cássia RFM (2010) Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci 25(3):423–430. https://doi.org/10.1007/s10103-009-0750-8
de Andrade ALM, Bossini PS, de Souza ALMC, Sanchez AD, Parizotto NA (2017) Effect of photobiomodulation therapy (808 nm) in the control of neuropathic pain in mice. Lasers Med Sci 32(4):865–872. https://doi.org/10.1007/s10103-017-2186-x
Cattin AL, Lloyd AC (2016) The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol 39:38–46. https://doi.org/10.1016/j.conb.2016.04.005
Funding
The authors thank FAPEMA (Foundation for Research Support and Scientific and Technological Development of the Maranhão) for the research grant support (Universal Process no. 01377/16), FAPESP (São Paulo Research Foundation) for the partial financial support (Grant no. 2009/01788-5), and CNPq (National Council for Scientific and Technological Development) for the productivity fellowship (Process no. 306344/2017-3). The authors also thank LACEMA (Academic League of Experimental Surgery of Maranhão).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
It is approved by Commission of Ethics in the Use of Animals (CEUA) from Federal University of Maranhão (protocol no. 23115.005396/2016-65).
Rights and permissions
About this article
Cite this article
de Almeida Melo Maciel Mangueira, M., Maciel Mangueira, N., Pereira Gama Filho, O. et al. Biochemical changes in injured sciatic nerve of rats after low-level laser therapy (660 nm and 808 nm) evaluated by Raman spectroscopy. Lasers Med Sci 34, 525–535 (2019). https://doi.org/10.1007/s10103-018-2627-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2627-1