Abstract
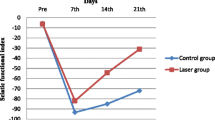
The study was aimed to validate the efficacy of the pulsed Nd:YAG laser on nerve regeneration in a rat sciatic nerve crushed model. 54 Wistar rats were randomly assigned into three groups: shame control, crush control, and laser treated group. For the laser treated group, the pulsed Nd:YAG laser (10 Hz) with 350 mJ per pulse in energy density and 50 J/cm2 in fluence was applied extracorporeally at the lesion site for 12 min to daily deliver 500 J immediately and consecutive 9 days following the crush injury. At week 1, the apoptosis-related activities in the injured nerve were examined (n = 8/each group). The sciatic functional index (SFI) was measured preoperatively and weekly until 4 weeks after the index procedure. The injured nerve and the innervated gastrocnemius muscle histology were assessed at week 4 (n = 10/each group). At week 1, the laser group showed the significant less TUNEL-positive ratio (P < 0.05), and the lower expression of cleaved caspase3/procaspase-3 and beclin-2/beclin-2-associated protein X ratios compared with the crush control. Furthermore, the laser group revealed significantly better SFI since week 1 and throughout the study (P < 0.05, all) compared with the crush control. At week 4, the laser group showed significantly higher axon density, lower myelin g-ratio, and the corresponding higher glycogen expression (P < 0.05, all) in the gastrocnemius muscle compared with those in the crush control. The pulsed Nd:YAG might enhance the injured nerve regeneration via apoptosis inhibition.





Similar content being viewed by others
Data Availability
All post analyzed data were showed in the main manuscript. The raw data generated during the current study are not publicly available due to the huge foot print data were collected, stored on the MATLAB program designed by our laboratory (MathWorks, Natick, MA) but are available from the corresponding author on reasonable request.
Abbreviations
- c-Cas3:
-
Cleaved caspase3
- p-Cas3:
-
Procaspase3
- BCL-2:
-
Beclin-2
- BAX:
-
Beclin-2-associated protein X
- SFI:
-
Sciatic functional index
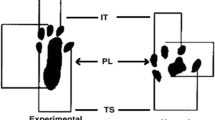
- PL:
-
Print length
- TS:
-
Toe spread
- IT:
-
Intermediary toe spread
References
Isaacs J (2010) Treatment of acute peripheral nerve injuries: current concepts. J Hand Surg Am 35:491–497
Garozzo D (2019) Peripheral nerve injuries and their surgical treatment: new perspectives on a changing scenario. Neurol India 67:S20–S22
Harhaus L, Hirche C, Giunta RE, Aszmann O, Siemers F, Kneser U, Lehnhardt M (2017) Strategies on the treatment of nerve injuries accompanied by severe soft tissue damage—Consensus statement of the German-speaking society for microsurgery of peripheral nerves and vessels. Handchir Mikrochir Plast Chir 49:257–266
Kaiser R (2016) Surgical treatment of lower extremity peripheral nerve injuries. Cas Lek Cesk 155:16–20
Li R, Liu Z, Pan Y, Chen L, Zhang Z, Lu L (2014) Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys 68:449–454
Topuz AK, Eroglu A, Atabey C, Cetinkal A (2013) Surgical treatment outcomes in peripheral nerve lesions due to gunshot injuries: assessment of 28 cases. Ulus Travma Acil Cerrahi Derg 19:235–240
Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martinez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, Jessen KR (2015) Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol 210:153–168
Koeppen AH (2004) Wallerian degeneration: history and clinical significance. J Neurol Sci 220:115–117
Liu X, Cui X, Guan G, Dong Y, Zhang Z (2020) microRNA-192-5p is involved in nerve repair in rats with peripheral nerve injury by regulating XIAP. Cell Cycle 19:326–338
Wang JB, Zhang Z, Li JN, Yang T, Du S, Cao RJ, Cui SS (2020) SPP1 promotes Schwann cell proliferation and survival through PKCalpha by binding with CD44 and alphavbeta3 after peripheral nerve injury. Cell Biosci 10:98
Zhao Z, Li X, Li Q (2017) Curcumin accelerates the repair of sciatic nerve injury in rats through reducing Schwann cells apoptosis and promoting myelinization. Biomed Pharmacother 92:1103–1110
Liu CY, Yin G, Sun YD, Lin YF, Xie Z, English AW, Li QF, Lin HD (2020) Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neurosci Ther 26:189–196
Kheshie AR, Alayat MS, Ali MM (2014) High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: a randomized controlled trial. Lasers Med Sci 29:1371–1376
Alayat MS, Mohamed AA, Helal OF, Khaled OA (2016) Efficacy of high-intensity laser therapy in the treatment of chronic neck pain: a randomized double-blind placebo-control trial. Lasers Med Sci 31:687–694
Alayat MSM, Alshehri MA, Shousha TM, Abdelgalil AA, Alhasan H, Khayyat OK, Al-Attar WS (2019) The effectiveness of high intensity laser therapy in the management of spinal disorders: a systematic review and meta-analysis. J Back Musculoskelet Rehabil 32:869–884
Alayat MSM, Basalamah MA, Elbarrany W, El-Sawy NAM, Abdel-Kafy EM, El-Fiky AA (2020) Dose-dependent effect of the pulsed Nd:YAG laser in the treatment of crushed sciatic nerve in Wister rats: an experimental model. Lasers Med Sci. https://doi.org/10.1007/s10103-020-02999-z
Lan SM, Yang CC, Lee CL, Lee JS, Jou IM (2017) The effect of molecular weight and concentration of hyaluronan on the recovery of the rat sciatic nerve sustaining acute traumatic injury. Biomed Mater 12:045024
Hong CK, Yeh ML, Chang CH, Chiang FL, Jou IM, Wang PH, Su WR (2019) Comparison of changes in shoulder functions between biceps tenotomy and tenodesis in an animal model. Asia Pac J Sports Med Arthrosc Rehabil Technol 15:17–22
Ko PY, Yang CC, Kuo YL, Su FC, Hsu TI, Tu YK, Jou IM (2018) Schwann-cell autophagy, functional recovery, and scar reduction after peripheral nerve repair. J Mol Neurosci 64:601–610
Lee HY, Hsieh TH, Liang JI, Yeh ML, Chen JJ (2012) Quantitative video-based gait pattern analysis for hemiparkinsonian rats. Med Biol Eng Comput 50:937–946
Liang JI, Chen MY, Hsieh TH, Liu CY, Lam CF, Chen JJ, Yeh ML (2012) Video-based gait analysis for functional evaluation of healing achilles tendon in rats. Ann Biomed Eng 40:2532–2540
Bain JR, Mackinnon SE, Hunter DA (1989) Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83:129–138
Rushton WA (1934) A physical analysis of the relation between threshold and interpolar length in the electric excitation of medullated nerve. J Physiol 82:332–352
Akgul T, Gulsoy M, Gulcur HO (2014) Effects of early and delayed laser application on nerve regeneration. Lasers Med Sci 29:351–357
dos Reis FA, Belchior AC, de Carvalho PT, da Silva BA, Pereira DM, Silva IS, Nicolau RA (2009) Effect of laser therapy (660 nm) on recovery of the sciatic nerve in rats after injury through neurotmesis followed by epineural anastomosis. Lasers Med Sci 24:741–747
Bae CS, Lim SC, Kim KY, Song CH, Pak S, Kim SG, Jang CH (2004) Effect of Ga-as laser on the regeneration of injured sciatic nerves in the rat. In Vivo 18:489–495
Shen CC, Yang YC, Huang TB, Chan SC, Liu BS (2013) Neural regeneration in a novel nerve conduit across a large gap of the transected sciatic nerve in rats with low-level laser phototherapy. J Biomed Mater Res A 101:2763–2777
Shen CC, Yang YC, Liu BS (2011) Large-area irradiated low-level laser effect in a biodegradable nerve guide conduit on neural regeneration of peripheral nerve injury in rats. Injury 42:803–813
Zhang LX, Tong XJ, Yuan XH, Sun XH, Jia H (2010) Effects of 660-nm gallium-aluminum-arsenide low-energy laser on nerve regeneration after acellular nerve allograft in rats. Synapse 64:152–160
Diker N, Aytac D, Helvacioglu F, Oguz Y (2020) Comparative effects of photobiomodulation therapy at wavelengths of 660 and 808 nm on regeneration of inferior alveolar nerve in rats following crush injury. Lasers Med Sci 35:413–420
Elsodany AM, Alayat MSM, Ali MME, Khaprani HM (2018) Long-term effect of pulsed Nd:YAG laser in the treatment of patients with rotator cuff tendinopathy: a randomized controlled trial. Photomed Laser Surg 36:506–513
Dundar U, Turkmen U, Toktas H, Solak O, Ulasli AM (2015) Effect of high-intensity laser therapy in the management of myofascial pain syndrome of the trapezius: a double-blind, placebo-controlled study. Lasers Med Sci 30:325–332
Gaudet AD, Popovich PG, Ramer MS (2011) Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 8:110
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Glushakova OY, Glushakov AO, Borlongan CV, Valadka AB, Hayes RL, Glushakov AV (2018) Role of caspase-3-mediated apoptosis in chronic caspase-3-cleaved tau accumulation and blood-brain barrier damage in the corpus callosum after traumatic brain injury in rats. J Neurotrauma 35:157–173
Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA (2005) Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci 25:3478–3487
Yang DP, Zhang DP, Mak KS, Bonder DE, Pomeroy SL, Kim HA (2008) Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: axon-dependent removal of newly generated Schwann cells by apoptosis. Mol Cell Neurosci 38:80–88
Acknowledgements
The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) guidelines of National Cheng Kung University (IACUC Approval No. 109263). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) for the reporting of animal experiments. No AI software was used in this manuscript writing. We are grateful to the Skeleton Materials and Bio-compatibility Core Lab, Clinical Medicine Research Center, National Cheng Kung University Hospital for the assistance in this study. We also wish to thank Ms. Yu-Ying Chen for her valuable assistance.
Funding
This study was supported by grant MOST108-2314-B-006-011-MY2, NSTC 112-2314-B-006-065, NSTC 112-2622-E-006-013, MOST 111-2314-B-006-054 from the National Science and Technology Council, Taiwan, and grant NCKUH-10904016, NCKUH-11004043 from the National Cheng Kung University Hospital, Tainan, Taiwan.
Author information
Authors and Affiliations
Contributions
P-YK, I-MJ, P-TW: conceptualization P-YK, C-CH, I-MJ, P-TW: methodology S-YC, C-LL: software C-CH, P-TW: validation P-YK, C-CH, S-YC, C-LL, I-MJ, P-TW: formal analysis P-YK, C-CH, P-TW, I-MJ: investigation S-YC, C-LL, I-MJ, P-TW: resources P-YK, C-CH, I-MJ, P-TW: data curation P-YK, C-CH, S-YC, C-LL, P-TW: writing—original draft preparation P-YK, C-CH, P-TW: writing—review and editing S-YC, C-LL, I-MJ: visualization P-YK, I-MJ, P-TW: supervision S-YC, C-LL, I-MJ, P-TW: project administration P-YK, I-MJ, P-TW: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
This research involving animals and the experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) guidelines of National Cheng Kung University (IACUC Approval No. 109263). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) for the reporting of animal experiments.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ko, PY., Hsu, CC., Chen, SY. et al. The Pulsed Nd:YAG Laser Therapy Enhanced Nerve Regeneration via Apoptosis Inhibition in a Rat Crushed Sciatic Nerve Model. Neurochem Res 49, 949–958 (2024). https://doi.org/10.1007/s11064-023-04068-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04068-7