Abstract
Background
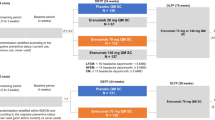
Erenumab is a monoclonal antibody acting against calcitonin gene-related peptide receptor which has been found effective even for the treatment of chronic migraine (CM) complicated with medication overuse headache (MOH). According to the present guidelines, the treatment with erenumab should continue for up to 1 year. The aim of the present study is to explore the evolution of patients affected by CM and MOH at the baseline, after erenumab discontinuation.
Methods
One hundred and eighty-five patients affected by CM and MOH were recruited and followed up after erenumab discontinuation. The number of migraine days per month, the number of painkillers taken per month, the number of days in which one medication was used for a month were collected every 30 days for the 3 months following erenumab suspension.
Results
At the 3rd month after suspension, patients displayed a significantly higher number of migraine days per month, a significantly higher painkiller consumption, and a significantly higher migraine-related disability. A high body mass index and the presence of aura were positively correlated with the relapse of CM and MOH.
Conclusion
Patients affected by CM and MOH at the baseline displayed a significant worsening of their headaches after erenumab discontinuation.



Similar content being viewed by others
Data availability
The dataset analyzed during the current study is available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- CGRP:
-
Calcitonin gene-related peptide
- CM:
-
Chronic migraine
- ICHD-3:
-
International Classification of Headache Disorders-3rd Edition
- MDM:
-
Migraine days per month
- MIDAS:
-
Migraine disability assessment questionnaire
- MOH:
-
Medication overuse headache
- NDM:
-
Number of days on medication
- NRS:
-
Numeric rating scale score
- PTPM:
-
Number of painkillers taken per month
References
Ashina M (2020) Migraine. N Eng J Med 383:1866–1876. https://doi.org/10.1056/NEJMra1915327
Headache Classification Committee of the International Headache Society (IHS) (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38:1–211. https://doi.org/10.1177/0333102417738202
Diener HC, Holle D, Solbach K, Gaul C (2016) Medication-overuse headache: risk factors, pathophysiology and management. Nat Rev Neurol 12:575–583. https://doi.org/10.1038/nrneurol.2016.124
GBD (2016) Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390:1211–1259. https://doi.org/10.1016/S0140-6736(17)32154-2
Chiang CC, Schwedt TJ, Wang SJ, Dodick DW (2016) Treatment of medication-overuse headache: a systematic review. Cephalalgia 36:371–381. https://doi.org/10.1177/0333102415593088
Sun-Edelstein C, Rapoport AM, Rattanawong W, Srikiatkhachorn A (2021) The evolution of medication overuse headache: history, pathophysiology and clinical update. CNS Drugs 35:545–565. https://doi.org/10.1007/s40263-021-00818-9
Cainazzo MM, Baraldi C, Ferrari A, Lo Castro F, Pani L, Guerzoni S (2021) Erenumab for the preventive treatment of chronic migraine complicated with medication overuse headache: an observational, retrospective, 12-month real-life study. Neurol Sci 42:4193–4202. https://doi.org/10.1007/s10072-021-05105-5
Sacco S, Bendtsen L, Ashina M, Reuter U, Terwindt G, Mitsikostas DD, Martelletti P (2019) European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain 20:6. https://doi.org/10.1186/s10194-018-0955-y
De Matteis E, Affaitati G, Frattale I, Caponnetto V, Pistoia F, Giamberardino MA, Sacco S, Ornello R (2021) Early outcomes of migraine after erenumab discontinuation: data from a real-life setting. Neurol Sci 42:3297–3303. https://doi.org/10.1007/s10072-020-05022-z
Gantenbein AR, Agosti R, Gobbi C, Flugel D, Schenkin CJ, Viceic D, Zecca C, Pohl H (2021) Impact on monthly migraine days of discontinuing anti-CGRP antibodies after one year of treatment - a real-life cohort study. Cephalalgia 41:1181–1186. https://doi.org/10.1177/03331024211014616
Raffaelli B, Terhart M, Overeem LH, Mecklenburg J, Neeb L, Steinicke M, Reuter U (2021) Migraine evolution after the cessation of CGRP(-receptor) antibody prophylaxis: a prospective, longitudinal cohort study. Cephalalgia; 3331024211046617. https://doi.org/10.1177/03331024211046617.
Yang M, Rendas-Baum R, Varon SF, Kosinski M (2011) Validation of the Headache Impact Test (HIT-6TM) across episodic and chronic migraine. Cephalalgia 31:357–367. https://doi.org/10.1177/0333102410379890
D’Amico D, Mosconi P, Genco S, Usai S, Prudenzano AMP, Grazzi L, Leone M, Puca FM, Bussone G (2001) The migraine disability assessment (MIDAS) questionnaire: translation and reliability of the Italian version. Cephalalgia 10:947–952. https://doi.org/10.1046/j.0333-1024.2001.00277.x
Raffaelli B, Mussetto V, Israel H, Neeb L, Reuter U (2019) Erenumab and galcanezumab in chronic migraine prevention: effects after treatment termination. J Headache Pain 20:66. https://doi.org/10.1186/s10194-019-1018-8
DeMatteis E, Guglielmetti M, Ornello R, Spuntarelli V, Martelletti P, Sacco S (2020) Targeting CGRP for migraine treatment: mechanisms, antibodies, small molecules, perspectives. Expert Rev Neurother 20:627–641. https://doi.org/10.1080/14737175.2020.1772758
Lee MJ, Lee SY, Cho S, Kang ES, Chung CS (2018) Feasibility of serum CGRP measurement as a biomarker of chronic migraine: a critical reappraisal. J Headache Pain 19:53. https://doi.org/10.1186/s10194-018-0883-x
Greco R, De Icco R, Demartini C, Zanaboni AM, Tumelero E, Sances G, Allena M, Tassorelli C (2020) Plasma levels of CGRP and espression of specific microRNAs in blood cells of episodic and chronic migraine subjects: towards the identification of a panel of peripheral biomarkers of migraine? J Headache Pain 21:122. https://doi.org/10.1186/s10194-020-01189-0
Tringali G, Vollono C, Calabresi P, Navarra P (2020) A proof-of-concept study on CGRP plasma levels of migraineurs during a 6-month treatment with ERENUMAB. J Headache Pain 21:124. https://doi.org/10.1186/s10194-020-01193-4
Bigal ME, Lipton RB (2011) Migraine chronification. Curr Neur Neurosci Rep 11:139–148. https://doi.org/10.1007/s11910-010-0175-6
Marics B, Peitl B, Varga A, Pàzmàndi K, Bàcsi A, Németh J, Szilvàssy Z, Jancsò G, Dux M (2017) Diet-induced obesity alters dural CGRP release and potentiates TRPA1-mediated trigeminovascular responses. Cephalalgia 37:581–591. https://doi.org/10.1177/0333102416654883
Vernieri F, Altamura C, Brunelli N, Maria Costa C, Aurilia C, Egeo G, Fofi L, Favoni V, Pierangeli G, Lovati C, Aguggia C, d’Onofrio F, Doretti A, Di Fiore P, Finocchi C, Rao R, Bono F, Ranieri A, Albanese M, Cevoli S, Barbanti P, GARLIT Study Group (2021) Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study). J Headache Pain 22:35. https://doi.org/10.1186/s10194-021-01247-1
Alpuente A, Gallardo VJ, Torres-Ferrùs M, Alavrez-Sabin J, Pozo-Rosich P (2020) Short and mid-term predictors of response to OnabotulinmtoxinA: real-life experience observational study. Headache 60:677–685
Tozzi A, De Iure A, Di Filippo M, Costa C, Caproni S, Pisani A, Bonsi P, Picconi B, Cupini LM, Materazzi S, Geppetti P, Sarchielli P, Calabresi P (2012) Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci 109:18985–18990
Close LN, Eftekhari S, Wang M, Charles AC, Russo AF (2019) Cortical spreading depression as a site of origin for migraine: role of CGRP. Cephalalgia 39:428–434
Baraldi C, Ornello R, Favoni V, Sacco S, Caponnetto V, Pierangeli G, Pani L, Cevoli S, Guerzoni S (2021) Chronic migraine and medication overuse headache worsening after OnabotulinumtoxinA withdrawn due to the severe acute respiratory syndrome-coronavirus-2 pandemic. Front Neurol 12:647995
Acknowledgements
None.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
LP, SG, and SC conceived and designed the study. Material preparation and data collection were performed by SG, BC, FLC, MMC, UP, VF, and SC. Data analysis was performed by CB and UP. The first draft of the manuscript was written by CB and reviewed by LP. All authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Area Vasta Emilia Nord Ethics Committee (protocol number: 50/2020/OSS/AOUMO) and by the Area Vasta Emilia Centro (protocol number:). All procedures were conducted in accordance with the latest version of the declaration of Helsinki.
Informed consent
Every subject signed an informed consent to participate in the study and for data publication.
Conflict of interest
CB received travel grants and honoraria from Allergan, Teva, Novartis, and Ely Lilly. VF received honoraria as a speaker or for participating in advisory boards from Ely Lilly, Novartis, and Teva. MMC received travel grants and honorary from Allergen, Novartis, Ibsa, and Ely Lilly. SC received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Novartis, Teva, Lilly, Allergan, Ibsa, and Lundbeck. SG received travel grants and honoraria from Allergan, Teva, Novartis, and Ely Lilly. LP is the Chief Scientific Officer of EDRA-LSWR Publishing Company and of Inpeco SA Total Lab Automation Company. In the last year he has been a scientific consultant to AbbVie, USA; BCG, Switzerland; Boehringer-Ingelheim, Germany; Compass Pathways, UK; Johnson & Johnson, USA; Takeda, USA; VeraSci, USA; Vifor, Switzerland. FLC and UP declare they have no conflict of interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guerzoni, S., Baraldi, C., Pensato, U. et al. Chronic migraine evolution after 3 months from erenumab suspension: real-world-evidence-life data. Neurol Sci 43, 3823–3830 (2022). https://doi.org/10.1007/s10072-022-05870-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-05870-x