Abstract
Background
Monoclonal antibodies targeting the calcitonin gene-related peptide, including erenumab, are migraine-specific preventive treatments, whose long-term effectiveness has still to be evaluated in real-life settings. We assessed early outcomes of erenumab discontinuation after a 52-week treatment in patients with a continuous positive response to the drug.
Methods
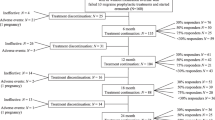
We evaluated the early outcomes after treatment completion in migraineurs from a real-life multicenter register. All patients received monthly erenumab for 52 weeks and attended a 8-week follow-up after treatment completion. Primary outcomes were responder rates and changes in monthly migraine days (MMDs), acute medications days (AMDs), and pain intensity on a Numerical Rating Scale (NRS score) during weeks 1–4 after erenumab treatment completion.
Results
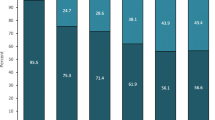
The 32 included patients reported a decrease in MMDs, AMDs, and NRS score during the last 4 weeks of treatment compared with baseline (P<0.001). During weeks 1–4 after treatment completion, all the outcome measures increased compared with the last 4 weeks of treatment (P < 0.001) despite staying lower than baseline (MMDs and AMDs P < 0.001, NRS score P = 0.005). Over the same time frame, 18 (56%) patients maintained a ≥ 50% reduction from baseline in MMDs. At week 4 after treatment completion, 10 (31%) patients restarted treatment due to disease rebound to baseline levels.
Conclusions
More than half patients had an early disease worsening, while the remaining patients maintained their responder status during weeks 1–4 after treatment completion. Further studies might identify predictors of prolonged response to erenumab and define the optimal treatment duration according to patients’ characteristics.



Similar content being viewed by others
Data availability
Source data of the study are available from the corresponding author on reasonable request.
References
(2019) Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):459–480. https://doi.org/10.1016/s1474-4422(18)30499-x
Stovner LJ, Andree C (2010) Prevalence of headache in Europe: a review for the Eurolight project. J Headache Pain 11(4):289–299. https://doi.org/10.1007/s10194-010-0217-0
Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB (2010) Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 30(9):1065–1072. https://doi.org/10.1177/0333102409355601
Martelletti P (2015) The therapeutic armamentarium in migraine is quite elderly. Expert Opin Drug Metab Toxicol 11(2):175–177. https://doi.org/10.1517/17425255.2015.982089
Martelletti P, Edvinsson L, Ashina M (2019) Shaping the future of migraine targeting calcitonin-gene-related-peptide with the disease-modifying migraine drugs (DMMDs). J Headache Pain 20(1):60. https://doi.org/10.1186/s10194-019-1009-9
Favoni V, Giani L, Al-Hassany L, Asioli GM, Butera C, de Boer I, Guglielmetti M, Koniari C, Mavridis T, Vaikjarv M, Verhagen I, Verzina A, Zick B, Martelletti P, Sacco S (2019) CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain 20(1):27. https://doi.org/10.1186/s10194-019-0979-y
Edvinsson L, Haanes KA, Warfvinge K, Krause DN (2018) CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol 14(6):338–350. https://doi.org/10.1038/s41582-018-0003-1
De Matteis E, Guglielmetti M, Ornello R, Spuntarelli V, Martelletti P, Sacco S (2020) Targeting CGRP for migraine treatment: mechanisms, antibodies, small molecules, perspectives. Expert Rev Neurother 20:627–641. https://doi.org/10.1080/14737175.2020.1772758
Agency EM (2019) Aimovig, INN-erenumab
Raffaelli B, Neeb L, Reuter U (2019) Monoclonal antibodies for the prevention of migraine. Expert Opin Biol Ther 19:1307–1317
Raffaelli B, Mussetto V, Israel H, Neeb L, Reuter U (2019) Erenumab and galcanezumab in chronic migraine prevention: effects after treatment termination. J Headache Pain 20(1):66. https://doi.org/10.1186/s10194-019-1018-8
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2014) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 12(12):1495–1499. https://doi.org/10.1016/j.ijsu.2014.07.013
Ornello R, Casalena A, Frattale I, Gabriele A, Affaitati G, Giamberardino MA, Asseta M, Maddestra M, Marzoli F, Viola S, Cerone D, Marini C, Pistoia F, Sacco S (2020) Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain 21:32
(2018) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38(1):1–211. https://doi.org/10.1177/0333102417738202
Sacco S, Bendtsen L, Ashina M, Reuter U, Terwindt G, Mitsikostas DD, Martelletti P (2019) Correction to: European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain 20(1):58. https://doi.org/10.1186/s10194-019-0972-5
May A, Schulte LH (2016) Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol 12(8):455–464. https://doi.org/10.1038/nrneurol.2016.93
Acknowledgments
The authors wish to thank all the study patients for their kind cooperation.
Author information
Authors and Affiliations
Contributions
RO and SS conceived the study and its design. EDM and RO performed the analysis and the interpretation of data. The first draft of the manuscript was written by EDM. Data were collected by IF, VC and GA. RO, SS, MAG, and FP revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
GA has received funds for congress participation from Innovet Italia Srl, Epitech Group, and Lusofarmaco; MAG received funds for congress participation from IBSA; SS has received speaking honoraria from and has served on Advisory Boards of Abbott, Allergan, Eli-Lilly, Medscape, Novartis, Teva; RO has received sponsorship to attend meetings from Novartis and Teva; EDM, IF, VC, and FP report no conflict of interest.
Ethics approval and consent to participate
The study was approved by the Internal Review Board of the University of L’Aquila (Italy), and patients gave written informed consent according to the Declaration of Helsinki.
Consent to publish
Not applicable.
Code availability
The study was approved by the Internal review Board of the University of L’Aquila with the number 44/2019.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 16.5 kb)
Rights and permissions
About this article
Cite this article
De Matteis, E., Affaitati, G., Frattale, I. et al. Early outcomes of migraine after erenumab discontinuation: data from a real-life setting. Neurol Sci 42, 3297–3303 (2021). https://doi.org/10.1007/s10072-020-05022-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-05022-z