Abstract
Introduction
Erenumab, a fully human monoclonal antibody against the calcitonin gene-related peptide receptor, is approved in Japan for the prevention of adult migraine. This post-hoc analysis evaluated the efficacy of erenumab in Japanese patients with low-frequency episodic migraine (LFEM) versus those with high-frequency episodic migraine (HFEM) and chronic migraine (CM).
Methods
A pooled analysis of data from the 24-week double-blind treatment phases (DBTPs) of phase 2 and 3 studies evaluated the efficacy of once-monthly erenumab 70 mg in Japanese patients. Patients were categorized into subgroups by monthly migraine days (MMD): LFEM and HFEM/CM. The main efficacy outcomes were change from baseline in MMD, acute migraine-specific medication treatment days (MSMD), and six-item Headache Impact Test (HIT-6™) scores.
Results
Patients with migraine (n = 532) were included in the analysis (LFEM, n = 215; HFEM, n = 215; CM, n = 102). Overall, mean age was 44 years, 86.5% were female, and 63.3–88.2% had used or were taking migraine preventive treatment at baseline. Throughout the DBTP, the placebo-adjusted mean change from baseline in MMD, MSMD, and HIT-6 scores with erenumab was similar across LFEM and HFEM/CM subgroups. The proportion of patients achieving at least 50% or 75% reduction from baseline in MMD and MSMD was similar across migraine frequency groups. Reduction in MMD moderately correlated with improvement in HIT-6 scores in the LFEM and HFEM/CM groups. Furthermore, the proportion of patients converting from HFEM/CM to LFEM during the DBTP was higher in the erenumab group than in the placebo.
Conclusion
In Japanese patients with different migraine frequencies, erenumab treatment resulted in significant improvements in MMD, MSMD, and headache impact. This pooled analysis of data from phase 2 and 3 studies increases confidence that erenumab is efficacious in patients with high MMD, which is associated with increased disability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Erenumab was previously demonstrated to be efficacious and safe in Japanese patients with episodic and chronic migraine. |
This post-hoc analysis of pooled data from the 24-week double-blind treatment phases of two clinical studies in Japan evaluated the efficacy of erenumab 70 mg across different migraine frequency groups. |
Treatment with erenumab resulted in significant reduction in monthly migraine days and acute migraine-specific medication treatment days, and improvement in headache impact scores across the migraine frequency groups. |
Erenumab is efficacious in Japanese patients with variable extent of migraine burden. |
Introduction
Migraine is a very common and debilitating neurological disorder with substantial impact on the affected individual and society [1,2,3]. Since 1990, migraine has increasingly been recognized as a major cause of disability worldwide [4, 5]. In 2016 and 2019, migraine was the second leading cause of years lived with disability worldwide and first and second among women and men, respectively, aged 15–49 years, which is an age group considered to be of peak productivity [4, 6].
In Japan, migraine affects approximately 6–8% of the population and is associated with a significant burden to patients and society in terms of health-related quality of life, work productivity, and health resource utilization and costs [7,8,9,10]. Use of acute medication among Japanese patients is widespread, while the current rate of adoption of oral preventive medications is low and associated with frequent discontinuation due to poor tolerability and inadequate or lack of efficacy [9, 11,12,13,14]. Since patients who derive meaningful benefits from treatment are more likely to persist on treatment than those without treatment benefits, it is important to capture the range of clinical benefits of migraine preventive treatment in patients with different levels of migraine frequency and disease burden [15].
Erenumab (erenumab-aooe in the United States) is a fully human monoclonal antibody that targets the canonical calcitonin gene-related peptide (CGRP) receptor. Erenumab is approved in the United States (2018), Europe (2018), and Japan (2021) for the preventive treatment of migraine in adults [16, 17]. Approval in Japan was based on two pivotal clinical trials evaluating the safety and efficacy of erenumab in adult Japanese patients with episodic migraine (EM) and chronic migraine (CM) [18,19,20]. Both studies demonstrated favorable safety and efficacy of erenumab in terms of reduction in monthly migraine days (MMD), reduction in monthly acute migraine-specific medication treatment days (MSMD), and reduction in measures of health-related quality of life.
Here, using pooled data from the 24-week, double-blind treatment phases (DBTPs) of phase 2 and phase 3 studies in Japan, we evaluated the efficacy of erenumab 70 mg based on reduction from baseline in MMD and MSMD in patients with low-frequency EM (LFEM; < 15 headache days/month; < 8 MMD) versus those with high-frequency EM (HFEM; < 15 headache days/month, ≥ 8 MMD) and CM (≥ 15 headache days/month; ≥ 8 MMD). We also evaluated patient-reported headache severity with the six-item Headache Impact Test (HIT-6™), the proportion of patients achieving at least 50% or 75% reduction in MMD and MSMD, the range of response to erenumab in individual patients, and the rate of conversion from one migraine subgroup at baseline to another during the DBTP. The purpose of this post-hoc analysis was to evaluate the benefits of erenumab for migraine prevention in patients with different frequencies of headaches/migraines.
Methods
Study Design
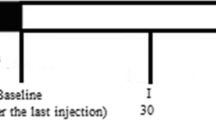
Efficacy data from the 24-week, placebo-controlled, DBTPs of phase 2 (NCT02630459) and phase 3 (NCT03812224) studies of erenumab for migraine prevention in Japan were used for a pooled analysis of patients with LFEM (< 15 headache days/month; < 8 MMD), HFEM (< 15 headache days/month; ≥ 8 MMD), and CM (≥ 15 headache days/month; ≥ 8 MMDs). Details of the study design for both studies have been previously described [19, 20]. The phase 2 study was done in adult Japanese patients with EM (< 15 headache days/month; ≥ 4 MMD) and included a screening phase (≤ 3 weeks), baseline phase (4 weeks), DBTP (24 weeks), open-label treatment phase (OLTP; 76 weeks), and a follow-up phase (12 weeks; Fig. 1). Patients received once-monthly placebo or erenumab 28 mg, 70 mg, or 140 mg in a 2:1:2:2 ratio. The phase 3 study was carried out in adult Japanese patients with EM or CM randomized 1:1 to once-monthly placebo or erenumab 70 mg. It included a screening phase (≤ 3 weeks), baseline phase (4 weeks), DBTP (24 weeks), OLTP (28 weeks), and a follow-up phase (8 weeks; Fig. 1). Only patients who received erenumab 70 mg during the DBTPs were included in this post-hoc pooled analysis, as this is the dose that is commercially approved in Japan. A migraine day was defined according to the International Classification of Headache Disorders, third edition of the International Headache Society, and consisted of any day during which a patient experienced a migraine with or without aura (≥ 4 h), and had two or more of the following pain features: unilateral, throbbing, moderate to severe, or worsened with physical activity; or associated with nausea, vomiting, or photophobia and phonophobia. A migraine day also included a day during which an acute migraine-specific medication was given to the patient regardless of the duration and associated symptoms. A headache day consisted of a day characterized by onset, continuation, or recurrence of a headache, and met one of the following criteria: a migraine headache treated with acute migraine-specific medication, a nonmigraine headache (≥ 4 h), or a headache for which acute headache treatment was used. An acute MSMD was any day during which migraine-specific medication (e.g., ergotamine derivatives, triptans) was used.
Study designs for the phase 2 and phase 3 studies aF/U visit was 16 weeks after the last dose of the IP for the phase 2 study and 12 weeks after the last dose of the IP for the phase 3 study. Only patients receiving ≥ 1 dose of erenumab 70 mg (orange boxes) or placebo (blue boxes) were included in the analysis. CM chronic migraine, DBTP double-blind treatment phase, EM episodic migraine, F/U follow-up, HFEM high-frequency episodic migraine, IP investigational product, LFEM low-frequency episodic migraine, MMD monthly migraine days, OLTP open-label treatment phase, QM once monthly, SC subcutaneous
Ethics committees or institutional review boards at each site reviewed and approved the protocol. All patients provided signed informed consent before study initiation. Oversight of study activities was conducted in conformity with appropriate regulatory requirements, including the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines of the International Council for Harmonisation.
Endpoints and Assessments
The primary endpoint of both studies was the change from baseline in mean MMD over months 4–6 of the DBTP. The efficacy outcomes of this post-hoc pooled analysis were change from baseline in MMD, MSMD, and HIT-6 scores at each month of the DBTP and at the mean of months 4–6; percent change in MMD and MSMD at each month of the DBTP and at the mean of months 4–6; proportion of patients who achieved at least 50% or 75% reduction from baseline in mean MMD and mean MSMD over months 4–6; and the proportion of patients converting from one migraine frequency subgroup at baseline to another during the DBTP.
Headache impact was evaluated with the HIT-6, a validated patient-reported outcome measure consisting of a six-item questionnaire evaluating the frequency of headache-related severe pain, limitations to usual activities, difficulty in concentration, desire to lie down, headache-related fatigue, and negative mood [21]. Higher HIT-6 scores represent a greater impact of headache; negative change from baseline indicates improvement in headache impact.
Statistical Analysis
The pooled post-hoc efficacy analysis set included Japanese patients with LFEM, HFEM, or CM who received at least one dose of erenumab 70 mg (as the first dose) or placebo. For the analyses of efficacy outcomes, patients were categorized into two groups based on MMD as follows: LFEM (< 8 MMD) versus HFEM and CM combined (HFEM/CM; ≥ 8 MMD). Combining patients with HFEM or CM into the same group for the analyses is based on the observation that these two migraine frequency subgroups, which are distinguished by the number of headache days/month (< 15 and ≥ 15, respectively), are similar with respect to disability, comorbidities, and disease burden [22,23,24]. Least-squares mean (LSM) at each time point is from a generalized linear mixed model, which includes treatment, visit, treatment-by-visit interaction, stratification factors of studies, prior migraine preventive treatment status (ever used or never used), and baseline value as covariates. A Cochran–Mantel–Haenszel test stratified by study and prior migraine preventive treatment status (ever or never) was used to estimate the common odds ratios (OR) and P values for achieving the response thresholds for reduction in mean MMD and mean MSMD in the erenumab 70 mg group versus placebo. Pearson correlation test was conducted to measure the linear relationship between the change from baseline in HIT-6 and MMD. Analyses were performed using SAS System 9.4 (SAS Institute, Cary, NC, USA). All P values reported are nominal without multiplicity adjustment.
Results
Patients and Baseline Characteristics
Of the 532 patients included in the post-hoc analysis set, 265 received erenumab 70 mg and 267 received placebo. Among all patients, 215 (40.4%) had LFEM, 215 (40.4%) had HFEM, and 102 (19.2%) had CM at baseline. Demographics and clinical characteristics at baseline were generally similar for the two treatment groups in all migraine frequency subgroups (Table 1). The mean age of patients was 43.5–44.9 years across treatment groups and migraine frequency subgroups; 84.3–92.0% were female. Most patients had used or were taking migraine preventive treatment at baseline, with a higher proportion of ever users among patients with HFEM (72.1%) or CM (88.2%) than among those with LFEM (63.3%). A majority of patients with LFEM (72.1%) and HFEM (61.4%) had never failed or were naïve to preventive treatment, whereas approximately 59.8%% of those with CM had failed at least one preventive treatment. The mean number of MMD was 5.9 days in LFEM patients, 9.9 days in HFEM patients, and 18.2 days in CM patients. The mean number of monthly headache days was 7.6 days in LFEM patients, 10.7 days in HFEM patients, and 20.9 days in CM patients. The mean number of MSMD was 4.4 days in LFEM patients, 7.5 days in HFEM patients, and 13.5 days in CM patients. Patient-reported outcome scores in HIT-6 were the lowest in patients with LFEM, followed by those with HFEM, and highest in those with CM; however, with baseline scores between 58 and 61 in the different migraine frequency subgroups, there is substantial impact to patients due to headache across all subgroups.
Change from Baseline in MMD, MSMD, and HIT-6 Scores in the LFEM and HFEM/CM Subgroups
At months 4–6, the LSM change from baseline in mean MMD in the overall DBTP population (LFEM and HFEM/CM) was –3.1 days in the erenumab 70 mg group and –1.1 days in the placebo group, for a difference [95% confidence interval (CI)] of –2.0 (–2.6, –1.5; P < 0.001) days (Fig. 2). The overall LSM change from baseline in mean MSMD was –2.0 days in the erenumab 70 mg group and –0.2 days in the placebo group, for a difference (95% CI) of –1.8 (–2.3, –1.3; P < 0.001) days (Figure S1). In general, the placebo-adjusted changes in MMD and MSMD with erenumab treatment in the LFEM subgroup were similar to those in the HFEM/CM subgroup during each month of the DBTP. At months 4–6, the placebo-adjusted LSM change from baseline (95% CI) in mean MMD with erenumab 70 mg was –1.9 (–2.6, –1.2; P < 0.001) days in the LFEM subgroup and –2.0 (–2.8, –1.2; P < 0.001) days in the HFEM/CM subgroup (Fig. 2). The placebo-adjusted LSM change from baseline (95% CI) in mean MSMD was –1.7 (–2.2, –1.1; P < 0.001) days in the LFEM subgroup and –1.8 (–2.5, –1.1; P < 0.001) days in the HFEM/CM subgroup (Figure S1). Although the placebo-adjusted changes in MMD and MSMD were similar between the migraine frequency subgroups with erenumab treatment, there was a higher percent change from baseline in the LFEM subgroup than in the HFEM/CM subgroup (Figure S2 and Figure S3).
LSM change from baseline in MMD during the DBTP. LSM is from an adjusted analysis utilizing a generalized linear mixed model, which includes treatment, visit, treatment-by-visit interaction, stratification factors of study (20120309 or 20170609) and prior migraine preventive treatment status (ever used or never used), and baseline value as covariates and assumes a first-order autoregression covariance structure. Nominal P values are shown without multiplicity adjustment. Δ, difference in treatment effect (erenumab 70 mg—placebo). Numbers in parentheses are the 95% CI values for the difference in treatment effect between erenumab 70 mg and placebo. Error bars represent SE. CI confidence interval, CM chronic migraine, DBTP double-blind treatment phase, HFEM high-frequency episodic migraine, LFEM low-frequency episodic migraine, LSM least-squares mean, MMD monthly migraine days, SE standard error of the mean
The overall placebo-adjusted LSM change from baseline (95% CI) in mean HIT-6 scores at months 4–6 with erenumab 70 mg was –1.6 points (–2.5, –0.8; P < 0.001; Fig. 4A), a between-group difference considered to be a clinically significant reduction in the impact of headache (≤ –1.5 points) [25]. Throughout the DBTP, the placebo-adjusted reductions in HIT-6 scores with erenumab in the migraine frequency subgroups were similar and ranged from –1.7 to –3.1 points in the LFEM subgroup and from –1.2 to –2.2 points in the HFEM/CM subgroup. At the patient level, 45.8% (119/260) of patients in the erenumab 70 mg group and 36.5% (96/263) of patients in the placebo group had a ≥ 5-point reduction from baseline in their HIT-6 scores (Figure S4B), which is considered to be a clinically significant reduction in headache impact at the individual level [26]. The proportion of patients achieving ≥ 5-point reduction from baseline in their HIT-6 scores with erenumab was similar in the LFEM (45.9%) and HFEM/CM (45.7%) subgroups, although the proportion of placebo responders was higher in the LFEM subgroup than in the HFEM/CM subgroup (40.5% vs. 33.6%).
Proportion of Patients Achieving ≥ 50% and ≥ 75% Reduction in MMD and MSMD in the LFEM and HFEM/CM Subgroups
At months 4–6, significantly more patients in the erenumab 70 mg group than in the placebo group achieved ≥ 50% and ≥ 75% reduction from baseline in mean MMD and mean MSMD in both LFEM and HFEM/CM subgroups (Fig. 3; Figure S5). In general, the proportions of MMD and MSMD responders with erenumab treatment in the LFEM subgroup were similar to those in the HFEM/CM subgroup. The placebo-adjusted response rate for ≥ 50% MMD reduction at months 4–6 [OR (95% CI)] was 14.3% [2.5 (1.2, 5.0); P = 0.009] for LFEM patients and 20.9% [4.1 (2.2, 7.6); P < 0.001] for HFEM/CM patients. At the ≥ 75% threshold for MMD reduction, the placebo-adjusted response rate was 8.1% [4.4 (1.2, 16.4); P = 0.02] for LFEM patients and 6.1% [5.9 (1.3, 27.1); P = 0.009] for HFEM/CM patients. The placebo-adjusted response rate for ≥ 50% MSMD reduction [OR (95% CI)] was 16.4% [4.1 (1.7, 9.5); P < 0.001] for LFEM patients and 15.9% [2.9 (1.6, 5.4), P < 0.001] for HFEM/CM patients (Figure S5). At the ≥ 75% threshold, the placebo-adjusted response rate was 8.1% [4.4 (1.2, 16.0); P = 0.02] for LFEM patients and 6.1% [5.9 (1.3, 27.1); P = 0.009] for HFEM/CM patients.
Proportion of patients achieving ≥ 50% and ≥ 75% reduction from baseline in MMD over months 4–6 of the DBTP. Common ORs and P values were obtained from a Cochran–Mantel–Haenszel test, stratified by stratification factors of study (20120309 or 20170609) and prior preventive treatment status (ever or never). Nominal P values were used for pairwise comparison without multiplicity adjustment. The P values for Breslow–Day tests of homogeneity of ORs across strata for ≥ 50% responders were 0.2 for LFEM and 0.7 for HFEM/CM, and for ≥ 75% responders were 0.9 for LFEM and 0.4 for HFEM/CM. CI confidence interval, CM chronic migraine, DBTP double-blind treatment phase, HFEM high-frequency episodic migraine, LFEM low-frequency episodic migraine, MMD monthly migraine days, OR odds ratio
Patient-Level Responses to Erenumab in the LFEM and HFEM/CM Subgroups
To gain insight into the range of response to erenumab based on reduction in MMD and HIT-6 scores in individual patients, waterfall plots and Pearson correlation analyses were performed. In general, patients with a higher baseline MMD tended to have a higher reduction in MMD at months 4–6 (Figure S6). In the LFEM subgroup, the proportion of patients with a reduction in MMD from baseline at months 4–6 was 73.7% (73/99) in the erenumab 70 mg group and 42.3% (47/111) in the placebo group. In the HFEM/CM subgroup, 86.1% (143/166) of patients in the erenumab 70 mg group and 71.7% (109/152) in the placebo group showed a reduction from baseline in MMD. Furthermore, there was a moderate correlation between the change from baseline in mean MMD and the change from baseline in mean HIT-6 scores over months 4–6 in patients overall (r < 0.5; P < 0.001), although the correlation was slightly stronger in the LFEM subgroup (r = 0.48) than in the HFEM/CM subgroup (r = 0.40; Fig. 4). The vast majority of patients who experienced a reduction in MMD also experienced an improvement in their HIT-6 scores at months 4–6; however, there were many patients who experienced an improvement in their HIT-6 scores without a reduction in MMD.
Scatter plot of change from baseline in mean MMD and mean HIT-6 scores over months 4–6 in individual patients treated with erenumab 70 mg. Pearson correlation analysis was carried out. The teal and blue dots represent patients with LFEM and HFEM/CM, respectively. CM chronic migraine, HFEM high-frequency episodic migraine, HIT-6 six-item Headache Impact test, LFEM low-frequency episodic migraine, MMD monthly migraine days, r Pearson correlation coefficient
Conversion Between LFEM and HFEM/CM
Next, we assessed the proportion of patients who converted from one migraine frequency subgroup at baseline to another during the DBTP. The proportion of patients who converted from HFEM/CM to LFEM during the DBTP was higher in the erenumab 70 mg group (44.8–55.8%) than in the placebo group (23.4–37.0%; Fig. 5). Conversely, the proportion of patients who converted from LFEM to HFEM/CM during the DBTP was lower in the erenumab 70 mg group (11.8–16.7%) than in the placebo group (29.2–42.5%).
Discussion
This post-hoc pooled efficacy analysis of two clinical studies of erenumab 70 mg in Japanese patients with migraine demonstrated that erenumab is efficacious across different migraine frequency subgroups. Overall, the efficacy of erenumab 70 mg compared to placebo based on change in MMD, MSMD, and HIT-6 scores was similar across the LFEM and HFEM/CM subgroups throughout the 24-week DBTP.
The proportion of patients with HFEM/CM who converted to LFEM during the DBTP was higher among erenumab-treated patients than it was for those treated with placebo. On the other hand, approximately 30% of LFEM patients treated with placebo converted to HFEM/CM during the DBTP, which suggests that early intervention with erenumab may be beneficial for these patients.
In general, erenumab treatment in patients with HFEM/CM resulted in greater reductions in MMD and MSMD than it did in patients with LFEM. However, after adjusting for the effect of the placebo group, the extent of reduction in MMD and MSMD was similar in the two subgroups. Considering the consistency in treatment effect with erenumab among patients with LFEM or HFEM/CM, they have a longer history of migraine, have more past treatment failures, and have more comorbidities than patients with LFEM, these results suggesting that erenumab treatment is beneficial across a wide range of migraine frequency, including patients with LFEM, HFEM, or CM. It may also suggest the importance of early intervention with CGRP preventive therapy for patients with LFEM.
Erenumab treatment also improved headache impact, as measured by HIT-6, throughout the DBTP for the vast majority of patients. In addition, most patients who experienced an improvement in headache impact also experienced a reduction in MMD. However, there were many patients who experienced an improvement in headache impact with little or no change in MMD, which suggests that treatment with erenumab can provide benefits beyond MMD reduction in patients with LFEM, HFEM, and CM. This moderate correlation between MMD reduction and improvement in headache impact was slightly stronger in the LFEM subgroup (r = 0.48) than in the HFEM/CM subgroup (r = 0.40), which may be due to outliers or the different distribution of change from baseline in MMD between the subgroups.
This post-hoc study has some limitations. The primary limitation is the variable placebo response for the efficacy outcomes in the two parent studies, which resulted in the variable placebo responses in the LFEM and HFEM/CM subgroups. In the phase 2 study, there was a negligible placebo response [19], whereas, in the phase 3 study, there was a notable placebo response similar to that observed in previous global studies of erenumab [20]. The reasons for the lack of a placebo response in the phase 2 study are unknown. It is possible that higher patient expectation due to the approval of other anti-CGRP pathway treatments, and prior knowledge of the favorable results from the phase 2 study by investigators and patients, may have contributed to the enhanced placebo response observed in the phase 3 study. Another limitation is that this pooled analysis is exploratory in nature and is not powered to detect statistically significant differences between the LFEM and HFEM/CM subgroups.
In summary, this post-hoc pooled analysis helps to further contextualize the benefits derived from erenumab treatment across different degrees of migraine frequency. The evidence presented herein is supportive of a favorable and clinically significant therapeutic effect of erenumab 70 mg compared to placebo on measures of migraine severity and impact across the range of migraine frequency types among Japanese patients.
Conclusion
In Japanese patients with different migraine frequencies, treatment with erenumab resulted in significant improvements in MMD, MSMD, and headache impact. By using a pooled analysis approach of data from the phase 2 and 3 studies, it was possible to make new observations increasing the confidence that erenumab is efficacious in patients with high MMD, which is associated with increased disability.
Data Availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available here: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request.
References
Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–9.
Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193–210.
Agosti R. Migraine burden of disease: from the patient’s experience to a socio-economic view. Headache. 2018;58(Suppl 1):17–32.
Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
Steiner TJ, Stovner LJ, Vos T. GBD 2015: migraine is the third cause of disability in under 50s. J Headache Pain. 2016;17(1):104.
Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Lifting the burden: the global campaign against H. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137.
Sakai F, Igarashi H. Prevalence of migraine in Japan: a nationwide survey. Cephalalgia. 1997;17(1):15–22.
Takeshima T, Ishizaki K, Fukuhara Y, Ijiri T, Kusumi M, Wakutani Y, et al. Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache. 2004;44(1):8–19.
Hirata K, Ueda K, Komori M, Zagar AJ, Selzler KJ, Nelson AM, et al. Comprehensive population-based survey of migraine in Japan: results of the ObserVational Survey of the Epidemiology, tReatment, and Care Of MigrainE (OVERCOME [Japan]) study. Curr Med Res Opin. 2021;37(11):1945–55.
Kikui S, Chen Y, Todaka H, Asao K, Adachi K, Takeshima T. Burden of migraine among Japanese patients: a cross-sectional National Health and Wellness Survey. J Headache Pain. 2020;21(1):110.
Meyers JL, Davis KL, Lenz RA, Sakai F, Xue F. Treatment patterns and characteristics of patients with migraine in Japan: a retrospective analysis of health insurance claims data. Cephalalgia. 2019;39(12):1518–34.
Blumenfeld AM, Bloudek LM, Becker WJ, Buse DC, Varon SF, Maglinte GA, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–55.
Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22–33.
Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478–88.
Tassorelli C, Diener HC, Dodick DW, Silberstein SD, Lipton RB, Ashina M, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38(5):815–32.
Aimovig (erenumab-aooe). Full Prescribing Information, Amgen, Inc., Thousand Oaks, CA, 2020.
Aimovig (erenumab). Summary of Product Characteristics. Dublin, Ireland: Novartis Europharm Limited; 2018.
Sakai F, Takeshima T, Tatsuoka Y, Hirata K, Cheng S, Numachi Y, et al. Long-term efficacy and safety during open-label erenumab treatment in Japanese patients with episodic migraine. Headache. 2021;61(4):653–61.
Sakai F, Takeshima T, Tatsuoka Y, Hirata K, Lenz R, Wang Y, et al. A randomized phase 2 study of erenumab for the prevention of episodic migraine in japanese adults. Headache. 2019;59(10):1731–42.
Takeshima T, Sakai F, Hirata K, Imai N, Matsumori Y, Yoshida R, et al. Erenumab treatment for migraine prevention in Japanese patients: efficacy and safety results from a Phase 3, randomized, double-blind, placebo-controlled study. Headache. 2021;61(6):927–35.
Houts CR, Wirth RJ, McGinley JS, Gwaltney C, Kassel E, Snapinn S, et al. Content validity of HIT-6 as a measure of headache impact in people with migraine: a narrative review. Headache. 2020;60(1):28–39.
Chalmer MA, Hansen TF, Lebedeva ER, Dodick DW, Lipton RB, Olesen J. Proposed new diagnostic criteria for chronic migraine. Cephalalgia. 2020;40(4):399–406.
Buse DC, Reed ML, Fanning KM, Bostic RC, Lipton RB. Demographics, headache features, and comorbidity profiles in relation to headache frequency in people with migraine: results of the American migraine prevalence and prevention (AMPP) study. Headache. 2020;60:2340–56.
Ishii R, Schwedt TJ, Dumkrieger G, Lalvani N, Craven A, Goadsby PJ, et al. Chronic versus episodic migraine: the 15-day threshold does not adequately reflect substantial differences in disability across the full spectrum of headache frequency. Headache. 2021;61(7):992–1003.
Smelt AF, Assendelft WJ, Terwee CB, Ferrari MD, Blom JW. What is a clinically relevant change on the HIT-6 questionnaire? An estimation in a primary-care population of migraine patients. Cephalalgia. 2014;34(1):29–36.
Bayliss M, Batenhorst AJL. RI: QualityMetric Incorporated. The HIT-6™ a user’s guide. 2002.
Acknowledgements
Medical Writing/Editorial Assistance
Medical writing support was provided by Qais Al-Hadid and Gillespie, Eugene (Amgen) and funded by Amgen.
Funding
This study was funded by Amgen and the Rapid Service Fee for this publication was paid by Amgen.
Author information
Authors and Affiliations
Contributions
Authors had full access to the study data and take full responsibility for the integrity and accuracy of the study data. Concept and design: Miki Hasebe, Daishi Yui. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: All authors. Critical revision for intellectual content: All authors. Statistical analysis: Cheng Peng, Reija Koukakis.
Corresponding author
Ethics declarations
Conflict of Interest
Shigekazu Kitamura and Takao Takeshima have nothing to disclose that could be construed as a conflict of interest. Reija Koukakis, Daishi Yui, Gabriel Paiva da Silva Lima, Cheng Peng, Ryuji Yoshida, Yotari Numachi, and Miki Hasebe are/were employees of Amgen. Reija Koukakis, Daishi Yui, Gabriel Paiva da Silva Lima, and Miki Hasebe disclose ownership of Amgen stock. Ryuji Yoshida is now affiliated with UCB Japan (Tokyo, Japan) and Yotaro Numachi is affiliated with Yu Mental Clinic (Tokyo, Japan).
Ethical Approval
This post-hoc analysis study involved human patients who provided informed consent for participation in the study that was approved by ethics committees and institutional review boards listed in the Supplementary Material. Oversight and conduct of the study were done in accordance with the ethical guidelines of the Declaration of Helsinki, Good Clinical Practice guidelines of the International Council for Harmonisation, and related regulations in Japan.
Additional information
Prior Publication Some of the results included here were presented at the 2022 and 2023 Japanese Society of Neurology (JSN) (Tokyo, Japan).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kitamura, S., Takeshima, T., Yui, D. et al. Efficacy of Erenumab for Migraine Prevention in Japanese Patients with Episodic and Chronic Migraine: Results of a Post-Hoc Pooled Analysis. Neurol Ther 12, 1993–2006 (2023). https://doi.org/10.1007/s40120-023-00538-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00538-w