Abstract
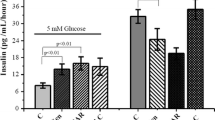
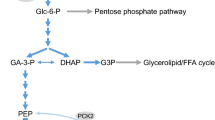
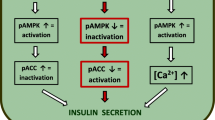
Genistein has been reported to potentiate glucose-stimulated insulin secretion (GSIS). Inhibitory activity on tyrosine kinase or activation of protein kinase A (PKA) was shown to play a role in the genistein-induced potentiation effect on GSIS. The aim of the present study was to elucidate the mechanism of genistein-induced potentiation of insulin secretion. Genistein augmented insulin secretion in INS-1 cells stimulated by various energy-generating nutrients such as glucose, pyruvate, or leucine/glutamine (Leu/Gln), but not the secretion stimulated by depolarizing agents such as KCl and tolbutamide, or Ca2+ channel opener Bay K8644. Genistein at a concentration of 50 μM showed a maximum potentiation effect on Leu/Gln-stimulated insulin secretion, but this was not sufficient to inhibit the activity of tyrosine kinase. Inhibitor studies as well as immunoblotting analysis demonstrated that activation of PKA was little involved in genistein-induced potentiation of Leu/Gln-stimulated insulin secretion. On the other hand, all the inhibitors of Ca2+/calmodulin kinase II tested, significantly diminished genistein-induced potentiation. Genistein also elevated the levels of [Ca2+]i and phospho-CaMK II. Furthermore, genistein augmented Leu/Gln-stimulated insulin secretion in CaMK II-overexpressing INS-1 cells. These data suggest that the activation of CaMK II played a role in genistein-induced potentiation of insulin secretion.
Similar content being viewed by others
References
Akiyama, T., Ishida, J., and Nakagawa, S. (1987). Genistein, a specific inhibitor of tyrosine-specific protein kinase. J. Biol. Chem. 262, 5592–5595.
Borge, P.D., Moibi, J., Greene, S.R., Trucco, M., Young, R.A., Gao, Z., and Wolf, B.A. (2002). Insulin receptor signaling and sarco/endoplasmic reticulum calcium ATPase in beta-cells. Diabetes 51, S427–S433.
Bratanova-Tochkova, T.K., Cheng, H,, Daniel, S., Gunawardana, S., Liu, Y.J., Mulvaney-Musa, J., Schermerhorn, T., Straub, S.G., Yajima, H., and Sharp, G.W. (2002). Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes 51, S83–S90.
Davies, S.P., Reddy, H., Caivano, M., and Cohen, P. (2000). Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105.
Eto, K., Yamashita, T., Tsubamoto, Y., Terauchi, Y., Hirose, K., Kubota, N., Yamashita, S., Taka, J., Satoh, S., Sekihara, H., et al. (2002). Phosphatidylinositol 3-kinase suppresses glucose-stimulated insulin secretion by affecting post-cytosolic [Ca(2+)] elevation signals Diabetes 51, 87–97.
Henquin, J.C. (2000). Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49, 751–760.
Jonas, J.C., Plant, T.D., Gilon, P., Detimary, P., Nenquin, M., and Henquin, J.C. (1995). Multiple effects and stimulation of insulin secretion by the tyrosine kinase inhibitor genistein in normal mouse islets. Br. J. Pharmacol. 114, 872–880.
Kang, K., Lee, S.B., Jung, S.H., Cha, K.H., Park, W.D., Sohn, Y.C., and Nho, C.W. (2009). Tectoridin, a poor ligand of estrogen receptor alpha, exerts its estrogenic effects via an ERK-dependent pathway. Mol. Cells 27, 351–357.
Knudsen, P., Kofod, H., Lernmark, A., and Hedeskov, C.J. (1983). L-leucine methyl ester stimulates insulin secretion and islet glutamate dehydrogenase. Am. J. Physiol. Endocrinol. Metab. 245, E338–E346.
Linassier, C., Pierre, M., Le Pecq, J.B., and Pierre, J. (1990). Mechanisms of action in NIH-3T3 cells of genistein, an inhibitor of EGF receptor tyrosine kinase activity. Biochem. Pharmacol. 39, 187–193.
Liu, Y.J., Cheng, H., Drought, H., MacDonald, M.J., Sharp, G.W., and Straub, S.G. (2003). Activation of the KATP channel-independent signaling pathway by the nonhydrolyzable analog of leucine, BCH. Am. J. Physiol. Endocrinol. Metab. 285, E380–E389.
Liu, D., Jiang, H., and Grange, R.W. (2005). Genistein activates the 3′,5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology 146, 1312–1320.
Liu, D., Zhen, W., Yang, Z., Carter, J.D., Si, H., and Reynolds, K.A. (2006). Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes 55, 1043–1050.
Maechler, P. (2002). Mitochondria as the conductor of metabolic signals for insulin exocytosis in pancreatic beta-cells. Cell. Mol. Life Sci. 59, 1803–1818.
Markiewicz, L., Garey, J., and Adlercreutz, H. (1993). In vitro bioassays of non-steroidal phytoestrogens. J. Steroi. Biochem. Mol. Biol. 45, 399–405.
Markovits, J., Linassier, C., Fossé, P., Couprie, J., Pierre, J., Jacquemin-Sablon, A., Saucier, J.M., Le Pecq, J.B., and Larsen, A.K. (1989). Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 49, 5111–5117.
Nesher, R., Anteby, E., Yedovizky, M., Warwar, N., Kaiser, N., and Cerasi, E. (2002). Beta-cell protein kinases and the dynamics of the insulin response to glucose. Diabetes 51, S68–S73.
Ng, W.W., Keung, W., Xu, Y.C., Ng, K.F., Leung, G.P., Vanhoutte, P.M., Choy, P.C., and Man, R.Y. (2008). Genistein potentiates protein kinase A activity in porcine coronary artery. Mol. Cell. Biochem. 311, 37–44.
Ohno, T., Kato, N., Ishii, C., Shimizu, M., Ito, Y., Tomono, S., and Kawazu, S. (1993). Genistein augments cyclic adenosine 3′5′- monophosphate(cAMP) accumulation and insulin release in MIN6 cells. Endocrine Res. 19, 273–285.
Ohsugi, M., Cras-Méneur, C., Zhou, Y., Bernal-Mizrachi, E., Johnson, J.D., Luciani, D.S., Polonsky, K.S., and Permutt, M.A. (2005). Reduced expression of the insulin receptor in mouse insulinoma (MIN6) cells reveals multiple roles of insulin signaling in gene expression, proliferation, insulin content, and secretion. J. Biol. Chem. 280, 4992–5003.
Persaud, S.J., Harris, T.E., Burns, C.J., and Jones, P.M. (1999) Tyrosine kinases play a permissive role in glucose-induced insulin secretion from adult rat islets. J. Mol. Endocrinol. 22, 19–28.
Sorenson, R.L., Brelje, T.C., and Roth, C. (1994). Effect of tyrosine kinase inhibitors on islets of langerhans: evidence for tyrosine kinases in the regulation of insulin secretion Endocrinology 134, 1975–1978.
Straub, S.G., and Sharp, G.W. (2002). Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab. Res. Rev. 18, 451–463.
Verspohl, E.J., Tollkühn, B., and Kloss, H. (1995). Role of tyrosine kinase in insulin release in an insulin secreting cell line (INS-1). Cell Signal. 7, 505–512.
Wan, Q.F., Dong, Y., Yang, H., Lou, X., Ding, J., and Xu, T. (2004). Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+. J. Gen. Physiol. 124, 653–662.
Wiederkehr, A., and Wollheim, C.B. (2006). Minireview: implication of mitochondria in insulin secretion and action. Endocrinology 147, 2643–2649.
Zawalich, W.S., and Zawalich, K.C. (2002). Effects of glucose, exogenous insulin, and carbachol on C-peptide and insulin secretion from isolated perifused rat islets. J. Biol. Chem. 277, 26233–26237.
Zawalich, W.S., Yamazaki, H., Zawalich, K.C., and Cline, G. (2004). Comparative effects of amino acids and glucose on insulin secretion from isolated rat or mouse islets. J. Endocrinol. 183, 309–319
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lee, SJ., Kim, HE., Choi, SE. et al. Involvement of Ca2+/calmodulin kinase II (CaMK II) in genistein-induced potentiation of leucine/glutamine-stimulated insulin secretion. Mol Cells 28, 167–174 (2009). https://doi.org/10.1007/s10059-009-0119-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-009-0119-7