Abstract
Pancreatic β-cells in the islets of Langerhans secrete insulin in response to blood glucose levels. Precise control of the amount of insulin secreted is of critical importance for maintaining systemic carbohydrate homeostasis. It is now well established that glucose induced production of ATP from ADP and the KATP channel closure elevate cytosolic Ca2+, triggering insulin exocytosis in β-cells. However, for full activation of insulin secretion by glucose, other mechanisms besides Ca2+ elevation are needed. These mechanisms are the targets of current research and include intracellular metabolic pathways branching from glycolysis. They are metabolic pathways originating from the TCA cycle intermediates, the glycerolipid/free fatty acid cycle and the pentose phosphate pathway. Signaling effects of these pathways including degradation (removal) of protein SUMOylation, modulation of insulin vesicular energetics, and lipid modulation of exocytotic machinery may converge to fulfill insulin secretion, though the precise mechanisms have yet to be elucidated. This mini-review summarize recent advances in research on metabolic coupling mechanisms functioning in insulin secretion.



Similar content being viewed by others
References
IDF Diabetes Atlas. Diabetes around the world in 2021. https://diabetesatlas.org.
Ahmed SM, Haris B, Saraswathi S, Elawwa A, Khalifa A, et al. The epidemiology, clinical, biochemical, immunological and radiological features of youth onset type 2 diabetes mellitus in the state of Qatar. Diabet Int. 2021. https://doi.org/10.1007/s13340-021-00548-9.
Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia. 2002;45:85–96.
Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10.
Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582:240–5.
Lane MA. The cytological characters of the areas of Langerhans. Am J Anatomy. 1907;7:409–22.
Hedeskov CJ. Mechanism of glucose-induced insulin secretion. Physiolo Rev. 1980;60:442–509.
Kojima I, Medina J, Nakagawa Y. Role of the glucose-sensing receptor in insulin secretion. Diabetes Obes Metab. 2017;19(Suppl 1):54–62.
Endo M. Reiji Natori, Setsuro Ebashi, and excitation-contraction coupling. Prog Biophys Mol Biol. 2011;105:129–33.
Calderón JC, Bolaños P, Caputo C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys Rev. 2014;6:133–60.
Penner R, Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988;139:329–45.
Maechler P, Kennedy ED, Pozzan T, Wollheim CB. Mitochondrial activation directly triggers the exocytosis of insulin in permeabilized pancreatic beta-cells. EMBO J. 1997;16:3833–41.
Kono T, Tong X, Taleb S, Bone RN, Iida H, et al. Impaired store-operated calcium entry and STIM1 loss lead to reduced insulin secretion and increased endoplasmic reticulum stress in the diabetic β-cell. Diabetes. 2018;67:2293–304.
Sato Y, Aizawa T, Komatsu M, Okada N, Yamada T. Dual functional role of membrane depolarization/Ca2+ influx in rat pancreatic B-cell. Diabetes. 1992;41:438–43.
Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992;89:1288–95.
Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, et al. Overexpression of hexokinase I but not GLUT1 glucose transporter alters concentration dependence of glucose-stimulated insulin secretion in pancreatic beta-cell line MIN6. J Biol Chem. 1994;269:3081–7.
Vionnet N, Stoffel M, Takeda J, Yasuda K, Bell GI, et al. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992;356:721–2.
Sekine N, Cirulli V, Regazzi R, Brown LJ, Gine E, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells potential low in nutrient sensing. J Biol Chem. 1994;269:4895–902.
Ishihara H, Wang H, Drewes LR, Wollheim CB. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in beta cells. J Clin Invest. 1999;104:1621–9.
Marqard J, Welters A, Bushmann T, Barthlen W, Vogelgesang S, et al. Association of exercise-induced hyperinsulinemic hypoglycemia with MCT1-expressing insulinoma. Diabetologia. 2013;56:31–5.
Zhao X, Capozzi ME, de Souza AH, Jahan I, Thomas CJ, et al. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab. 2020;32:736–50.
Abulizi A, Cardone RL, Stark R, Lewandowski SL, Zhao X, et al. Multi-tissue acceleration of the mitochondrial phosphoenolpyruvate cycle improves whole-body metabolic health. Cell Metab. 2020;32:751–66.
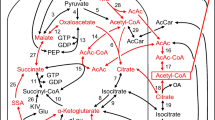
MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995;270:20051–8.
MacDonald MJ, Longacre MJ, Stoker SW, Kendrick M, Thonpho A, et al. Differences between human and rodent pancreatic islets. Low pyruvate carboxylase and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J Biol Chem. 2011;286:18383–96.
Soejima A, Inoue K, Takai D, Kaneko M, Ishihara H, et al. Mitochondrial DNA is required for regulation of glucose-stimulated insulin secretion in a mouse pancreatic beta cell line, MIN6. J Biol Chem. 1996;271:26194–9.
Silva JP, Köhler M, Graff C, Oldfors A, Magnuson MA, et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet. 2000;26:336–40.
van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, et al. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992;1:368–71.
Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, Veld P, et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–42.
Ronnebaum SM, Jensen MV, Hohmeier HE, Burgess SC, Zhou YP, et al. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem. 2008;283:28909–17.
Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose- stimulated insulin secretion. J Biol Chem. 2006;281:30593–602.
Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alárcon C, et al. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem. 2006;281:35624–32.
Zhang GF, Jensen MV, Gray SM, El K, Wang Y, et al. Reductive TCA cycle metabolism fuels glutamine- and glucose- stimulated insulin secretion. Cell Metab. 2021;33:804–17.
MacDonald MJ, Brown LJ, Longacre MJ, Stoker SW, Kendrick MA, et al. Knockdown of both mitochondrial isocitrate dehydrogenase enzymes in pancreatic beta cells inhibits insulin secretion. Biochem Biophys Acta. 2013;1830:5104–11.
Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, et al. Isocitrate- to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest. 2015;125:3847–60.
Li N, Zhang S, Xiong F, Eizrik DL, Wang CY. SUMOylation, a multifaceted regulatory mechanism in the pancreatic beta cells. Semin Cell Dev Biol. 2020;103:51–8.
Dai XQ, Plummer G, Casimir M, Kang Y, Hajkrle C, et al. SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans. Diabetes. 2011;60:838–47.
Bauchle CJ, Rohli KE, Boyer CK, Pal V, Rocheleau JV, et al. Mitochondrial efflux of citrate and isocitrate is fully dispensable for glucose-stimulated insulin secretion and pancreatic islet β-cell function. Diabetes. 2021;70:1717–28.
Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402:685–9.
Carobbio S, Ishihara H, Fernandez-Pascual S, Bartley C, Martin-Del-Rio R, Maechler P. Insulin secretion profiles are modified by overexpression of glutamate dehydrogenase in pancreatic islets. Diabetologia. 2004;47:266–76.
Casimir M, Lasorsa FM, Rubi B, Caille D, Palmieri F, et al. Mitochondrial glutamate carrier GC1 as a newly identified player in the control of glucose-stimulated insulin secretion. J Biol Chem. 2009;284:25004–14.
Gheni G, Ogura M, Iwasaki M, Yokoi N, Minami K, et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 2014;23:661–73.
Dyachok O, Idevall-Hagren O, Sagetorp J, Tian G, Wuttke A, et al. Glucose-induced cAMP oscillations regulate pulsatile insulin secretion. Cell Metab. 2008;8:26–37.
Gammelsaeter R, Coppola T, Marcaggi P, Storm-Mathisen J, Chaudhry FA, et al. A role for glutamate transporters in the regulation of insulin secretion. PLoS ONE. 2011;6:e22960.
Zhou Y, Waanders LF, Holmseth S, Guo C, Berger UV, et al. Proteome analysis and conditional deletion of the EAAT2 glutamate transporter provide evidence against a role of EAAT2 in pancreatic insulin secretion in mice. J Biol Chem. 2014;2(89):1329–44.
Jesinkey SR, Madiraju AK, Alves TC, Yarborough OH, Cardone RL, et al. Mitochondrial GTP links nutrient sensing to β cell health, mitochondrial morphology, and insulin secretion independent of OxPhos. Cell Rep. 2019;28:759–72.
Cantley J, Davenport A, Vetterli L, Nemes NJ, Whitworth PT, et al. Disruption of beta cell acetyl-CoA carboxylase-1 in mice impairs insulin secretion and beta cell mass. Diabetologia. 2019;62:99–111.
Mugabo Y, Zhao S, Seifried A, Gezzar S, Al-Mass A, et al. Identification of a mammalian glycerol-3-phosphate phosphatase: role in metabolism and signaling in pancreatic beta-cells and hepatocytes. Proc Natl Acad Sci U S A. 2016;113:E430–9.
Kwan EP, Xie L, Sheu L, Nolan CJ, Prentki M, et al. Munc13-1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes. 2006;55:1421–9.
Kang L, He Z, Xu P, Fan J, Betz A, et al. Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metab. 2006;3:463–8.
Zhao S, Mugabo Y, Iglesias J, Xie L, Delghingaro-Augusto V, et al. α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 2014;19:993–1007.
Spegel P, et al. Time-resolved metabolomics analysis of beta-cells implicates the pentose phosphate pathway in the control of insulin release. Biochem J. 2013;450:595–605.
Gooding JR, et al. Adenylosuccinate is an insulin secretagogue derived from glucose- induced purine metabolism. Cell Rep. 2015;13:157–67.
Prentki M, Corkey BE, Madiraju SRM. Lipid-associated metabolic signaling networks in pancreatic beta cell function. Diabetologia. 2020;63:10–20.
Campbell JE, Newgard CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol. 2021;22:142–58.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HI received a research grant from Astellas and donations from Boehringer Ingelheim, Daiichi Sankyo, Mitsubishi Tanabe, Eli-Lilly, and lecture fees from Novo Nordisk and Merck Sharp and Dohme.
Ethics statement
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ishihara, H. Metabolism-secretion coupling in glucose-stimulated insulin secretion. Diabetol Int 13, 463–470 (2022). https://doi.org/10.1007/s13340-022-00576-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-022-00576-z