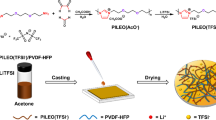
Abstract
A series of waterborne polyurethane (WPU) with various hard segment and soft segment content were synthesized by hexamethylene diisocyanate (HDI), polyethylene glycol (PEG), dimethylol propionic acid (DMPA), and diethylene glycol (DEG). An increase in hard segment content decreased the crystallinity and thermal stability of WPU. Solid polymer electrolytes (SPEs) were prepared by complexing the as-prepared WPU with LiTFSI. The ionic conductivity increased first with the increasing of the hard segment content and then decreased. The compatibility of WPU-based SPEs with lithium electrode was influenced by the hard segment content. The electrochemical stability windows for all the SPEs have reached around 5.0 V (vs. Li+/Li) at 60 °C. A maximum ion conductivity of 5.14 × 10−5 S cm−1 at 25 °C was found for WPU12-20%Li and 1.29 × 10−3 S cm−1 at 60 °C for WPU12-25%Li. All-solid-state LiFePO4/SPE/Li battery based on WPU12-20%Li electrolyte delivered discharge specific capacities of 159 and 162 mAh g−1 under 60 and 80 °C at 0.1 C, respectively. Tuning the appropriate hard and soft segment composition of WPU may ultimately lead to the successful use of WPU-based SPEs for all-solid-state lithium ion batteries.








Similar content being viewed by others
References
Shim J, Kim DG, Kim HJ, Lee JH, Lee JC (2015) Polymer composite electrolytes having core–shell silica fillers with anion-trapping boron moiety in the shell layer for all-solid-state lithium-ion batteries. ACS Appl Mater Interfaces 7(14):7690–7701. https://doi.org/10.1021/acsami.5b00618
Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J (2017) Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33:363–386. https://doi.org/10.1016/j.nanoen.2017.01.028
Wang A, Xu H, Zhou Q, Liu X, Li Z, Gao R, Liu X, Zhang L (2017) Electrochemical performances of a new solid composite polymer electrolyte based on hyperbranched star polymer and ionic liquid for lithium-ion batteries. J Solid State Electrochem 21(8):2355–2364. https://doi.org/10.1007/s10008-017-3582-7
Swiderska-Mocek A, Jakobczyk P, Lewandowski A (2017) Kinetic and galvanostatic studies of a polymer electrolyte for lithium-ion batteries. J Solid State Electrochem 21(10):2825–2831. https://doi.org/10.1007/s10008-017-3609-0
Long L, Wang S, Xiao M, Meng Y (2016) Polymer electrolytes for lithium polymer batteries. J Mater Chem A 4(26):10038–10069. https://doi.org/10.1039/C6TA02621D
Yan X, Li Z, Wen Z, Han W (2017) Li/Li7La3Zr2O12/LiFePO4 all-solid-state battery with ultrathin nanoscale solid electrolyte. J Phys Chem C 121(3):1431–1435. https://doi.org/10.1021/acs.jpcc.6b10268
He D, Cho SY, Kim DW, Lee C, Kang Y (2012) Enhanced ionic conductivity of semi-IPN solid polymer electrolytes based on star-shaped oligo(ethyleneoxy)cyclotriphosphazenes. Macromolecules 45(19):7931–7938. https://doi.org/10.1021/ma3016745
Ma T, Yu X, Cheng X, Li H, Zhu W, Qiu X (2017) Confined solid electrolyte interphase growth space with solid polymer electrolyte in hollow structured silicon anode for Li-Ion batteries. ACS Appl Mater Interfaces 9(15):13247–13254. https://doi.org/10.1021/acsami.7b03046
Zhai H, Xu P, Ning M, Cheng Q, Mandal J, Yang Y (2017) A flexible solid composite electrolyte with vertically aligned and connected ion-conducting nanoparticles for lithium batteries. Nano Lett 17(5):3182–3187. https://doi.org/10.1021/acs.nanolett.7b00715
Vélez JF, Aparicio M, Mosa J (2016) Covalent silica-PEO-LiTFSI hybrid solid electrolytes via sol-gel for Li-ion battery applications. Electrochim Acta 213:831–841. https://doi.org/10.1016/j.electacta.2016.07.146
Zhen R, Chi Q, Wang X, Yang K, Jiang Y, Li FF, Xue B (2016) Crystallinity, ion conductivity, and thermal and mechanical properties of poly(ethylene oxide)-illite nanocomposites with exfoliated illite as a filler. J Appl Polym Sci 133(47):4426. https://doi.org/10.1002/APP.44226
Polu AR, Rhee HW (2017) Ionic liquid doped PEO-based solid polymer electrolytes for lithium-ion polymer batteries. Int J Hydrog Energy 42(10):7212–7219. https://doi.org/10.1016/j.ijhydene.2016.04.160
Xue Z, Heb D, Xie X (2015) Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J Mater Chem A 3(38):19218–19253. https://doi.org/10.1039/C5TA03471J
Shim J, Lee JW, Bae KY, Kim HJ, Yoon WY, Lee JC (2017) Dendrite suppression by synergistic combination of solid polymer electrolyte crosslinked with natural terpenes and lithium-powder anode for lithium-metal batteries. ChemSusChem 10(10):2274–2283. https://doi.org/10.1002/cssc.201700408
Shim J, Kim L, Kim HJ, Jeong D, Lee JH, Lee JC (2017) All-solid-state lithium metal battery with solid polymer electrolytes based on polysiloxane crosslinked by modified natural gallic acid. Polymer 122:222–231. https://doi.org/10.1016/j.polymer.2017.06.074
Tsao CH, Ueda M, Kuo PL (2016) Synthesis and characterization of polymer electrolytes based on cross-linked phenoxy-containing polyphosphazenes. J Polym Sci Part A: Polym Chem 54(3):352–358. https://doi.org/10.1002/pola.27781
Liu X, Ding G, Zhou X, Li S, He W, Chai J, Pang C, Liu Z, Cui G (2017) An interpenetrating network poly(diethylene glycol carbonate)-based polymer electrolyte for solid state lithium batteries. J Mater Chem A 5(22):11124–11130. https://doi.org/10.1039/C7TA02423A
He W, Cui Z, Liu X, Cui Y, Chai J, Zhou X, Liu Z, Cui G (2017) Carbonate-linked poly(ethylene oxide) polymer electrolytes towards high performance solid state lithium batteries. Electrochim Acta 225:151–159. https://doi.org/10.1016/j.electacta.2016.12.113
Liu L, Wu X, Li T (2014) Novel polymer electrolytes based on cationic polyurethane with different alkyl chain length. J Power Sources 249:397–404. https://doi.org/10.1016/j.jpowsour.2013.10.116
Mustapa SR, Aung MM, Ahmad A, Mansor A, TianKhoon L (2016) Preparation and characterization of jatropha oil-based polyurethane as non-aqueous solid polymer electrolyte for electrochemical devices. Electrochim Acta 222:293–302. https://doi.org/10.1016/j.electacta.2016.10.173
Khatoon H, Ahmad S (2017) A review on conducting polymer reinforced polyurethane composites. J Ind Eng Chem 53:1–22. https://doi.org/10.1016/j.jiec.2017.03.036
Karimi MB, Khanbabaei G, Sadeghi GMM (2017) Vegetable oil-based polyurethane membrane for gas separation. J Membr Sci 527:198–206. https://doi.org/10.1016/j.memsci.2016.12.008
Lee YH, Kim JS, Noh J, Lee I, Kim HJ, Choi S, Seo J, Jeon S, Kim TC, Lee JY, Choi JW (2013) Wearable textile battery rechargeable by solar energy. Nano Lett 13(11):5753–5761. https://doi.org/10.1021/nl403860k
Milroy C, Manthiram AA (2016) An elastic, conductive, electroactive nanocomposite binder for flexible sulfur cathodes in lithium-sulfur batteries. Adv Mater 28(44):9744–9751. https://doi.org/10.1002/adma.201601665
Liu K, Liu M, Cheng J, Dong S, Wang C, Wang Q, Zhou X, Sun H, Chen X, Cui G (2016) Novel cellulose/polyurethane composite gel polymer electrolyte for high performance lithium batteries. Electrochim Acta 215:261–266. https://doi.org/10.1016/j.electacta.2016.08.076
Porcarelli L, Manojkumar K, Sardon H, Llorente O, Shaplov AS, Vijayakrishna K, Gerbaldi C, Mecerreyes M (2017) Single ion conducting polymer electrolytes based on versatile polyurethanes. Electrochim Acta 241:526–534. https://doi.org/10.1016/j.electacta.2017.04.132
Chang Z, Zhang M, Hudson AG, Orler EB, Moore RB, Wilkes GL, Turner SR (2013) Synthesis and properties of segmented polyurethanes with triptycene units in the hard segment. Polymer 54(26):6910–6917. https://doi.org/10.1016/j.polymer.2013.10.028
Wang S, Jeung S, Min K (2010) The effects of anion structure of lithium salts on the properties of in-situ polymerized thermoplastic polyurethane electrolytes. Polymer 51(13):2864–2871. https://doi.org/10.1016/j.polymer.2010.04.022
Wang S, Min K (2010) Solid polymer electrolytes of blends of polyurethane and polyether modified polysiloxane and their ionic conductivity. Polymer 51(12):2621–2628. https://doi.org/10.1016/j.polymer.2010.04.038
Bao J, Tao C, Yu R, Gao M, Huang Y, Chen CH (2017) Solid polymer electrolyte based on waterborne polyurethane for all-solid-state lithium ion batteries. J Appl Polym Sci 134(48):45554. https://doi.org/10.1002/app.45554
Tan R, Gao R, Zhao Y, Zhang M, Xu J, Yang J, Pan F (2016) Novel organic–inorganic hybrid electrolyte to enable LiFePO4 quasi-solid-state Li-ion batteries performed highly around room temperature. ACS Appl Mater Interfaces 8(45):31273–31280. https://doi.org/10.1021/acsami.6b09008
Hood MA, Wang B, Sands JM, Scala JJL, Beyer FL, Li CY (2010) Morphology control of segmented polyurethanes by crystallization of hard and soft segments. Polymer 51(10):2191–2198. https://doi.org/10.1016/j.polymer.2010.03.027
Moins S, Martins JC, Krumpmann A, Lemaur V, Cornil J, Delbosc N, Decroly A, Dubois P, Lazzaroni R, Gohy JF, Coulembier O (2017) Chem Commun 53(51):6899–6902. https://doi.org/10.1039/C7CC02385E
Zhao Y, Huang Z, Chen S, Chen B, Yang J, Zhang Q, Ding F, Chen Y, Xu X (2016) A promising PEO/LAGP hybrid electrolyte prepared by a simple method for all-solid-state lithium batteries. Solid State Ionics 295:65–71. https://doi.org/10.1016/j.ssi.2016.07.013
Skarja GA, Woodhouse KA (2000) Structure-property relationships of degradable polyurethane elastomers containing an amino acid-based chain extender. J Appl Polym Sci 75(12):1522–1534. https://doi.org/10.1002/(SICI)1097-4628(20000321)75:12<1522::AID-APP11>3.0.CO;2-A
Ramesh S, Liew CW (2013) Dielectric and FTIR studies on blending of [xPMMA–(1−x)PVC] with LiTFSI. Measurement 46(5):1650–1656. https://doi.org/10.1016/j.measurement.2013.01.003
Bar N, Basak P, Tsur Y (2017) Vibrational and impedance spectroscopic analyses of semi-interpenetrating polymer networks as solid polymer electrolytes. Phys Chem Chem Phys 19(22):14615–14624. https://doi.org/10.1039/C7CP00129K
Prabakaran P, Manimuthu RP, Gurusamy S (2017) Influence of barium titanate nanofiller on PEO/PVdF-HFP blend-based polymer electrolyte membrane for Li-battery applications. J Solid State Electrochem 21(5):1273–1285. https://doi.org/10.1007/s10008-016-3477-z
Zhou X, Fang C, Lei W, Su J, Li L, Li Y (2017) Thermal and Crystalline Properties of Waterborne Polyurethane by in situ water reaction process and the potential application as biomaterial. Prog Org Coat 104:1–10. https://doi.org/10.1016/j.porgcoat.2016.12.001
Yoshimoto N, Nomura H, Shirai T, Ishikawa M, Morita M (2004) Ionic conductance of gel electrolyte using a polyurethane matrix for rechargeable lithium batteries. Electrochim Acta 50(2-3):275–279. https://doi.org/10.1016/j.electacta.2004.01.128
Panwiriyarat W, Tanrattanakul V, Pilard JF, Pasetto P, Khaokong C (2013) Effect of the diisocyanate structure and the molecular weight of diols on bio-based polyurethanes. J Appl Polym Sci 130(1):453–462. https://doi.org/10.1002/app.39170
Zhang J, Ma C, Xia Q, Liu J, Ding Z, Xu M, Chen L, Wei W (2016) Composite electrolyte membranes incorporating viscous copolymers with cellulose for high performance lithium-ion batteries. J Membr Sci 497:259–269. https://doi.org/10.1016/j.memsci.2015.09.056
Zhang H, Liu C, Zheng L, Xu F, Feng W, Li H, Huang X, Armand M, Nie J, Zhou Z (2014) Lithium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolyte. Electrochim Acta 133:529–538. https://doi.org/10.1016/j.electacta.2014.04.099
Yen MS, Kuo SC (1998) PCL-PEG-PCL triblock ester-ether copolydiol-based waterborne polyurethane. II. Effect of NCO/OH mole ratio and DMPA content on the physical properties. J Appl Polym Sci 67(7):1301–1311. https://doi.org/10.1002/(SICI)1097-4628(19980214)67:7<1301::AID-APP21>3.0.CO;2-2
Wen TC, Wang YJ, Cheng TT, Yang CH (1999) Polymer 14:3979–3988
Ciosek M, Sannier L, Siekierski M, Golodnitsky D, Peled E, Scrosati B, Glowinkowski S (2007) Electrochim Acta 53(4):1409–1416. https://doi.org/10.1016/j.electacta.2007.03.037
Verma ML, Minakshi M, Singh NK (2014) Synthesis and characterization of solid polymer electrolyte based on activated carbon for solid state capacitor. Electrochim Acta 137:497–503. https://doi.org/10.1016/j.electacta.2014.06.039
Ng STC, Forsyth M, MacFarland DR, Garcia M, Smith ME, Strange JH (1998) Composition effects in polyetherurethane-based solid polymer electrolytes. Polymer 39(25):6261–6268. https://doi.org/10.1016/S0032-3861(98)00153-0
Wen TC, Wu MS, Yang CH (1999) Spectroscopic investigations of poly(oxypropylene)glycol-based waterborne polyurethane doped with lithium perchlorate. Macromolecules 32(8):2712–2720. https://doi.org/10.1021/ma9804489
Ferry A, Jacobsson P, Heumen JDV, Stevens JR (1996) Raman, infra-red and d.s.c. studies of lithium coordination in a thermoplastic polyurethane. Polymer 37(5):737–744. https://doi.org/10.1016/0032-3861(96)87248-X
Zhang J, Ma C, Liu J, Chen L, Pan A, Wei W (2016) Solid polymer electrolyte membranes based on organic/inorganic nanocomposites with star-shaped structure for high performance lithium ion battery. J Membr Sci 509:138–148. https://doi.org/10.1016/j.memsci.2016.02.049
Choudhary S, Sengwa RJ (2017) Effects of different inorganic nanoparticles on the structural, dielectric and ion transportation properties of polymers blend based nanocomposite solid polymer electrolytes. Electrochim Acta 247:924–941. https://doi.org/10.1016/j.electacta.2017.07.051
Korley LTJ, Pate BD, Thomas EL, Hammond PT (2006) Effect of the degree of soft and hard segment ordering on the morphology and mechanical behavior of semicrystalline segmented polyurethanes. Polymer 47(9):3073–3082. https://doi.org/10.1016/j.polymer.2006.02.093
Hossieny N, Shaayegan V, Ameli A, Saniei M, Park CB (2017) Characterization of hard-segment crystalline phase of thermoplastic polyurethane in the presence of butane and glycerol monosterate and its impact on mechanical property and microcellular morphology. Polymer 112:208–218. https://doi.org/10.1016/j.polymer.2017.02.015
Hu P, Duan Y, Hu D, Qin B, Zhang J, Wang Q, Liu Z, Cui G, Chen L (2015) Rigid-flexible coupling high ionic conductivity polymer electrolyte for an enhanced performance of LiMn2O4/graphite battery at elevated temperature. ACS Appl Mater Interfaces 7(8):4720–4727. https://doi.org/10.1021/am5083683
Wetjen M, Kim GT, Joost M, Appetecchi GB, Winter M, Passerini S (2014) Thermal and electrochemical properties of PEO-LiTFSI-Pyr14TFSI-based composite cathodes, incorporating 4 V-class cathode active materials. J Power Sources 246:846–857. https://doi.org/10.1016/j.jpowsour.2013.08.037
Xu C, Sun B, Gustafsson T, Edstrom K, Brandell D, Hahlin M (2014) Interface layer formation in solid polymer electrolyte lithium batteries: an XPS study. J Mater Chem A 2(20):7256–7264. https://doi.org/10.1039/C4TA00214H
Chen B, Xu Q, Huang Z, Zhao Y, Chen S, Xu X (2016) One-pot preparation of new copolymer electrolytes with tunable network structure for all-solid-state lithium battery. J Power Sources 331:322–331. https://doi.org/10.1016/j.jpowsour.2016.09.063
Yang C, Fu K, Zhang Y, Hitz E, Hu L (2017) Adv Mater 29:36. https://doi.org/10.1002/adma.201701169
Cheng XB, Zhang R, Zhao CZ, Zhang Q (2017) Toward safe lithium metal anode in rechargeable batteries: a review. Chem Rev 117(15):10403–10473. https://doi.org/10.1021/acs.chemrev.7b00115
Guo Y, Li H, Zhai T (2017) Reviving lithium-metal anodes for next-generation high-energy batteries. Adv Mater 29:1700007. https://doi.org/10.1002/adma.201700007
Acknowledgements
This study was supported by Education Ministry of Anhui Province (KJ2017A031), and we gratefully acknowledge the Engineering Technology Research Center of Waterborne Polymer Materials of Anhui Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 242 kb)
Rights and permissions
About this article
Cite this article
Ren, N., Song, Y., Tao, C. et al. Effect of the soft and hard segment composition on the properties of waterborne polyurethane-based solid polymer electrolyte for lithium ion batteries. J Solid State Electrochem 22, 1109–1121 (2018). https://doi.org/10.1007/s10008-017-3855-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3855-1