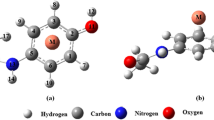
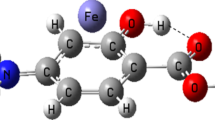
Abstract
In order to reveal the essence of the pharmaceutical incompatibility, the cooperativity effects of the drug–drug intermolecular π∙∙∙π and H∙∙∙O H-bonding interactions involving hydration were evaluated in the phenobarbital∙∙∙paracetamol∙∙∙H2O complex at the M06-2X/6–311++G** and MP2/6–311++G** levels. The thermodynamic cooperativity effects were also investigated by the statistical thermodynamic method. The results show that the π∙∙∙π stacking ternary complexes with the moderate anti-cooperativity effects are dominant in controling the aggregation process of phenobarbital, paracetamol, and H2O, as is confirmed by the atoms-in-molecules (AIM) and reduced density gradient (RDG) analyses. Therefore, it can be inferred that the anti-cooperativity effect plays an important role in forming the pharmaceutical incompatibility, and thus a deduction on the formation process of the pharmaceutical incompatibility between phenobarbital and paracetamol, with the hydration effect, is given. Several valuable models that relate the features of molecular surface electrostatic potentials or their statistical parameters, such as the surface areas, average values (\( \overline{V_s} \)), variances (\( {\sigma}_{\mathrm{tot}}^2 \), \( {\sigma}_{+}^2 \) and \( {\sigma}_{-}^2 \)), and product of \( {\sigma}_{\mathrm{tot}}^2 \) and electrostatic balance parameter (ν) (\( {\sigma}_{\mathrm{tot}}^2 \)ν), to the values of the cooperativity effects were predicted. The formation of the pharmaceutical incompatibility is a thermodynamic cooperativity process driven by the enthalpy change.

Anti-cooperativity effect plays an important role in forming the pharmaceutical incompatibility








Similar content being viewed by others
References
Thanacoody H (2012) Drug interactions. In: Walker R, Whittlesea C (Eds.) Clinical pharmacy and therapeutics, 5th edn. Elsevier, Amsterdam, p 50–61
Conde-Estévez D (2017) Targeted cancer therapy: interactions with other medicines. Clin Transl Oncol 19:21–30
Wf Van Leeuwen R, Swart EL, Boom FA, Schuitenmaker MS, Hugtenburg JG (2010) Potential drug interactions and duplicate prescriptions among ambulatory cancer patients: a prevalence study using an advanced screening method. BMC Canc 10:679
Hadjibabaie M, Badri S, Ataei S, Moslehi AH, Karimzadeh I, Ghavamzadeh A (2013) Potential drug–drug interactions at a referral hematology-oncology ward in Iran: a cross-sectional study. Canc Chemother Pharmacol 71:1619–1627
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Somerfield MR, Lyman GH (2017) Antiemetics: American society of clinical oncology clinical practice guideline update. J Clin Oncol 35:3240–3261
Lopez-Martin C, Garrido Siles M, Alcaide-Garcia J, Faus Felipe V (2014) Role of clinical pharmacists to prevent drug interactions in cancer outpatients: a single-Centre experience. Int J Clin Pharm 36:1251–1259
Riechelmann RP, Tannock IF, Wang L, Saad ED, Taback NA, Krzyzanowska MK (2007) Potential drug interactions and duplicate prescriptions among cancer patients. J Natl Cancer Inst 99:592–600
Popa MA, Wallace KJ, Brunello A, Extermann M, Balducci L (2014) Potential drug interactions and chemotoxicity in older patients with cancer receiving chemotherapy. J Geriatr Oncol 5:307–314
Stockley IH (2002) Drug interactions6th edn. The Pharmaceutical Press, London
Politzer P, Murray JS, Clark T (2015) Mathematical modeling and physical reality in noncovalent interactions. J Mol Model 21:52
Clark T, Murray JS, Politzer P (2018) A perspective on quantum mechanics and chemical concepts in describing noncovalent interactions. Phys Chem Chem Phys 20:30076–30082
Mignon P, Loverix S, Steyaert J, Geerlings P (2005) Influence of the π-π interaction on the hydrogen bonding capacity of stacked DNA/RNA bases. Nucl Acids Res 33:1779–1789
Hesselmann A, Jansen G, Schutz M (2006) Interaction energy contributions of H-bonded and stacked structures of the AT and GC DNA base pairs from the combined density functional theory and intermolecular perturbation theory approach. J Am Chem Soc 128:11730–11731
Leist R, Frey JA, Ottiger P, Frey HM, Leutwyler S, Bachorz RA, Klopper W (2007) Nucleobase-fluorobenzene interactions: hydrogen bonding wins over π-stacking. Angew Chem Int Ed 46:7449–7452
Hasanzadeh M, Shadjou N (2016) Pharmacogenomic study using bio- and nanobioelectrochemistry: drug–DNA interaction. Mat Sci Eng C-Mater 61:1002–1017
Poornima CS, Dean PM (1995) Hydration in drug design. 3. Conserved water molecules at the ligand-binding sites of homologous proteins. J Comput Aid Mol Des 9:521–531
Falgun S, Jiri G, Jennifer L, Devleena S, Woody S, Philip JR, Mitchell AA (2012) Computer-aided drug design of falcipain inhibitors: virtual screening, structure−activity relationships, hydration site thermodynamics, and reactivity analysis. J Chem Inf Model 52:696–710
Cheema MA, Taboada P, Barbosa S, Siddiq M, Mosquera V (2006) Effect of molecular structure on the hydration of structurally related antidepressant drugs. Mol Phys 104:3203–3212
Geist L, Mayer M, Cockcroft XL, Wolkerstorfer B, Kessler D, Engelhardt H, McConnell DB, Konrat R (2017) Direct NMR probing of hydration shells of protein ligand interfaces and its application to drug design. J Med Chem 60:8708–8715
Ayesha Z, Jóhannes R (2016) Hydration free energy as a molecular descriptor in drug design: a feasibility study. Mol Inf 35:207–214
Hausman DS, Cambron RT, Sakr A (2005) Application of on-line Raman spectroscopy for characterizing relationships between drug hydration state and tablet physical stability. Int J Pharm 299:19–33
Marini A, Berbenni V, Bruni G, Cofrancesco P, Margheritis C, Orlandi A, Villa M (2004) Hydration, stability, and phase transformations of a new antitumor drug. J Pharm Sci 93:2222–2231
Souza MS, Diniz LF, Vogt L, Carvalho Jr PS, D’vries RF, Ellena J (2018) Mechanochemical synthesis of a multicomponent solid form: the case of 5-fluorocytosine isoniazid codrug. Cryst Growth Des 18:5202–5209
Vijay D, Sastry GN (2010) The cooperativity of cation-π and π-π interactions. Chem Phys Lett 485:235–242
Garcia-Raso A, Albertí FM, Fiol JJ, Tasada A, Barceló-Oliver M, Molins E, Escudero D, Frontera A, Quiñonero D, Deyà PM (2007) Anion-π interactions in bisadenine derivatives: a combined crystallographic and theoretical study. Inorg Chem 46:10724–10735
Alkorta I, Blanco F, Deyà PM, Elguero J, Estarellas C, Frontera A, Quiñonero D (2010) Cooperativity in multiple unusual weak bonds. Theor Chim Acta 126:1–14
Hunter CA, Anderson HL (2009) What is cooperativity? Angew Chem Int Ed Engl 48:7488–7499
Guengerich FP (1999) Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 39:1–17
Denisov IG, Frank DJ, Sligar SG (2009) Cooperative properties of cytochromes P450. Pharmacol Ther 124:151–167
Domanski TL, He Y-A, Khan KK, Roussel F, Wang Q, Halpert JR (2001) Phenylalanine and tryptophan scanning mutagenesis of CYP3A4 substrate recognition site residues and effect on substrate oxidation and cooperativity. Biochemistry 40:10150–10160
Frank DJ, Denisov IG, Sligar SG (2009) Mixing apples and oranges: analysis of heterotropic cooperativity in cytochrome P450 3A4. Arch Biochem Biophys 488:146–152
Müller CS, Knehans T, Davydov DR, Bounds PL, von Mandach U, Halpert JR, Caflisch A, Koppenol WH (2015) Concurrent cooperativity and substrate inhibition in the epoxidation of carbamazepine by cytochrome P450 3A4 active site mutants inspired by molecular dynamics simulations. Biochemistry 54:711–721
Hlavica P (2017) Challenges in assignment of allosteric effects in cytochrome P450-catalyzed substrate oxidations to structural dynamics in the hemoprotein architecture. J Inorg Biochem 167:100–115
Castrignanò S, D’Avino S, Nardo GD, Catucci G, Sadeghi SJ, Gilardi G (2018) Modulation of the interaction between human P450 3A4 and B. megaterium reductase via engineered loops. BBA-Proteins Proteom 1866:116–125
Du H, Li J, Cai Y, Zhang H, Liu G, Tang Y, Li W (2017) Computational investigation of ligand binding to the peripheral site in CYP3A4: conformational dynamics and inhibitor discovery. J Chem Inf Model 57:616–626
Lappin G, Shishikura Y, Jochemsen R, Weaver RJ, Gesson C, Houston B, Oosterhuis B, Bjerrum OJ, Rowland M, Garner C (2010) Pharmacokinetics of fexofenadine: evaluation of a microdose and assessment of absolute oral bioavailability. Eur J Pharm Sci 40:125–131
Lappin G, Shishikura Y, Jochemsen R, Weaver RJ, Gesson C, Oosterhuis JB, Oosterhuis B, Bjerrum OJ, Grynkiewicz G, Alder J, Rowland M, Garner C (2011) Comparative pharmacokinetics between a microdose and therapeutic dose for clarithromycin, sumatriptan, propafenone, paracetamol (acetaminophen), and phenobarbital in human volunteers. Eur J Pharm Sci 43:141–150
Tian Q-P, Song S-Q, Shi W-J, Xie Y, Song Y-H, Tang H-F, Gong M-X (2014) Investigation on the percutaneous enhancing permeation mechanism of azone for ketoprofen based on the intermolecular hydrogen-bonding interaction. Chinese J Struc Chem 33:304–318
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc., Wallingford
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Bader RFW (1990) Atoms in molecules, a quantum theory. Oxford University Press, Oxford
Lu T, Chen F (2012) Quantitative analysis of molecular surface based on improved marching tetrahedra algorithm. J Mol Graphics Modell 38:314–323
Duijineveldt FB, Duijineveldt-van de Rijdt JCMV, Lenthe JHV (1994) State of the art in counterpoise theory. Chem Rev 94:1873–1885
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the difference of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Day GM, Motherwell WDS, Jones WA (2007) Strategy for predicting the crystal structures of flexible molecules: the polymorphism of phenobarbital. Phys Chem Chem Phys 9:1693–1704
Zencirci N, Griesser UJ, Gelbrich T, Kahlenberg V, Jetti RKR, Apperley DC, Harris RK (2014) New solvates of an old drug compound (phenobarbital): structure and stability. J Phys Chem B 118:3267–3280
Wilson CC (1997) Neutron diffraction of p-hydroxyacetanilide (paracetamol): libration or disorder of the methyl group at 100 K. J Mol Struct 405:207–217
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew Chem Int Ed 34:1555–1573
Gelbrich T, Rossi D, Häfele CA, Griesser UJ (2011) Barbiturates with hydrogen-bonded layer and framework structures. CrystEngComm 13:5502–5509
An GW, Zhang H, Cheng X-L, Zhuo Q-L, Lv Y-C (2008) Electronic structure and hydrogen bond in the crystal of paracetamol drugs. Struct Chem 19:613–617
Schleyer PR, Maerker C, Dransfeld A, Jiao H, van Eikema Hommes NJR (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318
Qin XF, Wu HS, Jiao H (2007) Structure and stability of closo-BnHn−2(CO)2(n=5–12). J Mol Struct (THEOCHEM) 810:135–141
Debeer EL, Bottone AE, Voest EE (2001) Doxorubicin mechanical performance cardiactrabeculae after acute chronictreatment: review. Eur J Pharmacol 415:1–11
Soniat M, Rogers DM, Rempe SB (2015) Dispersion-and exchange-corrected density functional theory for sodium ion hydration. J Chem Theory Comput 11:2958–2967
DiLabio GA, Johnson ER, Otero-de-la-Roza A (2013) Performance of conventional and dispersion-corrected density-functional theory methods for hydrogen bonding interaction energies. Phys Chem Chem Phys 15:12821–12828
Zabardasti A, Zare N, Arabpour M (2011) Theoretical study of dihydrogen bonded clusters of water with tetrahydroborate. Struct Chem 22:691–695
Jiang L, Bai P, Wang J, Liu B, Li Y (2018) Experimental and theoretical insight into the cooperativity effect in composite wax powder and ternary complex of coronene with CH4 and Mn+ (Mn+ = Li+, Na+, K+, Be2+, Mg2+ or Ca2+). Mol Phys 166:143–153
Murray JS, Politzer P (2011) The electrostatic potential: an overview. WIREs Comput Mol Sci 1:153–163
Q-p T, Wang Y-h, W-j S, S-q S, H-f T (2013) A theoretical investigation into the cooperativity effect between the H∙∙∙O and H∙∙∙F− interactions and electrostatic potential upon the 1:2 (F−:N-(Hydroxymethyl)acetamide) ternary-system formation. J Mol Model 19:5171–5185
James F, Berry B, Nadezhda VK, Yegor DS, Emna MNP, Vladimir IP (2011) NMR structures of apo L. casei dihydrofolate reductase and its complexes with trimethoprim and NADPH: contributions to positive cooperative binding from ligand-induced refolding, conformational changes, and interligand hydrophobic interactions. Biochemistry 50:3609–3620
Birdsall B, Burgen ASV, Roberts GCK (1980) Binding of coenzyme analogues to Lactobacillus casei dihydrofolate reductase: binary and ternary complexes. Biochemistry 19:3723–3731
Williams DH, Stephens E, O’Brien DP, Zhou M (2004) Understanding non-covalent interactions: ligand binding energy and catalytic efficiency from ligand-induced reductions in motion within receptors and enzymes. Angew Chem Int Ed 43:6596–6616
G-r F, T-y Q, W-j S, Guo Y-x, Y-j Z, Guo J, L-x K (2014) A B3LYP and MP2(full) theoretical investigation on the cooperativity effect between hydrogen-bonding and cation-molecule interactions and thermodynamic property in the 1: 2 (Na+: N-(Hydroxymethyl)acetamide) ternary complex. J Mol Model 20:2154
Calderone CT, Williams DH (2001) An enthalpic component in cooperativity: the relationship between enthalpy, entropy, and noncovalent structure in weak associations. J Am Chem Soc 123:6262–6267
Andrea F, Jack G (2012) Enthalpy–entropy compensation and cooperativity as thermodynamic epiphenomena of structural flexibility in ligand–receptor interactions. J Mol Biol 417:454–467
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
We allow the journal to review all the data, and confirm the validity of the results. We have none of the financial relationships. This manuscript was not published previously and it is not submitted to more than one journal. This work is not split up into several parts to submit. No data have been fabricated or manipulated.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Selected geometric parameters, intermolecular interaction energies, plots of the RDG versus the electron density multiplied by the sign of the second Hessian eigenvalue of the binary complexes as well as the bond paths of AIM results for the ternary complexes are collected in Supplementary data.
ESM 1
(DOC 543 kb)
Rights and permissions
About this article
Cite this article
Zhai, Fp., Wei, He., Liu, Y. et al. Theoretical explanation for the pharmaceutical incompatibility through the cooperativity effect of the drug–drug intermolecular interactions in the phenobarbital∙∙∙paracetamol∙∙∙H2O complex. J Mol Model 25, 181 (2019). https://doi.org/10.1007/s00894-019-4060-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4060-1