Abstract
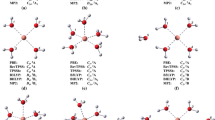
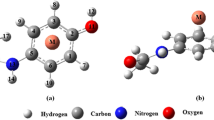
Knowledge of coordination modes of metal and pharmaceuticals are crucial for gaining understanding of the chemical mechanisms underlying their biological activity, in particular for systems where there is a synergism based on the fact that complexes can provide enhanced activity of the drug with fewer side effects. Quantum chemistry calculations represent a unique and complementary approach to experimental methods to understand the thermodynamics of reactions in terms of structural details of the participating species. Here, the coordination modes between Cu(II) and piroxicam and their stability constants were studied, by means of DFT molecular modeling, in gas phase and in solution (water and ethanol) at the RevTPSS/def-SVP and (SMD- and CPCM-RevTPSS)/def-SVP levels of theory, respectively. Octahedral bidentate geometries are found to be the more stable, likely due to the chelate effect. Thermodynamic results on the stability of the formed complexes revealed that complexation is favored in ethanol. The calculated logK s with the (SMD-RevTPSS)/def-SVP level of theory are in better agreement to the experimental values than (CPCM-RevTPSS)/def-SVP results.






Similar content being viewed by others
References
Page J, Henry D (2000) Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Int Med 160:777–784
McGeer PL, Schilzer M, McGeer EG (1996) Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology 47:425–432
Sugaya K, Uz T, Kumar V, Manev H (2000) New anti-inflammatory treatment strategy in Alzheimer’s disease. Jpn J Pharmacol 82:85–94
Jain AK (2008) Solubilization of indomethacin using hydrotropes for aqueous injection. Eur J Pharm Biopharm 68:701–714
Williams PAM, Molinuevo MS, Okulik N, Jubert AH, Etcheverry S (2005) Synthesis, characterization and biological properties of vanadyl (IV) complexes of diclofenac and indomethacin: an experimental and theoretical study. Appl Organomet Chem 19:711–718
McNally R (2001) Drug information for health care professional, 21st edn. USP–DI, Massachusetts
Moncada S, Vane JR (1979) Mode of action of aspirin-like drugs. Adv Int Med 24:1–22
Mamett LJ, Kalgutkar AS (1999) Cyclooxygenase 2 inhibitors: discovery, selectivity and the future. Trends Pharmacol Sci 20:465–469
Weder JE, Dillon CT, Hambley TW, Kennedy BJ, Lay PA, Biffin JR, Regtop HL, Davies NM (2002) Copper complexes of non-steroidal anti-inflammatory drugs: an opportunity yet to be realized. Coord Chem Rev 232:95–126
Christofis P, Katsarou M, Papakyriakou A, Sanakis Y, Katsaros N, Psomas G (2005) Mononuclear metal complexes with piroxicam: synthesis, structure and biological activity. J Inorg Biochem 99:2197–2210
Ho J, Coote ML, Franco-Pérez M, Gómez-Balderas R (2010) First-principles prediction of the pKas of anti-inflammatory oxicams. J Phys Chem A 114:11992–12003
Milanino R, Mauro U, Marrella M, Pasqualicchio M, Gaspe − Rini R, Velo G (1995) In: Berthon G (ed) Handbook of metal-ligand interactions in biological fluids. Marcel Decker, New York
Sorenson JRJ (1989) Copper complexes offer a physiological approach to treatment of chronic diseases. Prog Med Chem 26:437–568
Tagliati CA, Kimura E, Nothenberg MS, Santos SRJC, Oga S (1999) Pharmacokinetic profile and adverse gastric effect of zinc-piroxicam in rats. Gen Pharmacol 33:67–71
Samara CD, Tsotsou G, Ekateriniadou LV, Kortsaris AH, Raptopoulou CP, Terzis A, Kyriakidis DA, Kersissoglou DP (1998) Anti-inflammatory drugs interacting with Zn(II), Cd(II) and Pt(II) metal ions. J Inorg Biochem 71:171–179
Rodríguez-Laguna N, Reyes-García LI, Moya-Hernández R, Rojas-Hernández A, Gómez-Balderas (2016) Chemical speciation of the system Cu(II)-indomethacin in ethanol and water by UV–Vis spectrophotometry. J Chem. doi:10.1155/2016/9804162
Cini R, Giorgi G, Cinquantini A, Rossi C, Sabat M (1990) Metal complexes of the anti-inflammatory drug piroxicam. Inorg Chem 29:5197–5200
Gehad GM, El-Gamel NEA (2004) Preparation and spectroscopic characterization of metal complexes of piroxicam. Vib Spectrosc 36:97–104
Gehad GM (2005) Structural and thermal characterization of ternary complexes of piroxicam and alanine with transition metals: uranyl binary and ternary complexes of piroxicam Spectroscopic characterization and properties of metal complexes. Spectrochim Acta A 62:1165–1171
Roy S, Banerjee R, Sarkar M (2006) Direct binding of Cu(II)-complexes of oxicam NSAIDs with DNA backbone. J Inorg Biochem 100:1320–1331
Tamasi G, Serinelli F, Consumi M, Magnani A, Casolaro M, Cini R (2008) Release studies from smart hydrogels as carriers for piroxicam and copper(II)-oxicam complexes as anti-inflammatory and anti-cancer drugs. X-ray structures of new copper(II)-piroxicam and -isoxicam complex molecules. J Inorg Biochem 102:1862–1873
Hadadzadeh H, Salimi M, Weil M, Jannesari Z, Darabi F, Abdi K, Khalaji AD, Sardari S, Ahangari R (2012) The piroxicam complex of copper(II), trans-[Cu(Pir)2(THF)2], and its interaction with DNA. J Mol Struct 1022:172–180
Abu-Eittah RH, Zordok WA (2010) A molecular orbital treatment of piroxicam and its M2+-complexes: the change of the drug configuration in a time of bond formation. J Mol Struct Theochem 951:14–20
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc., Wallingford CT
GaussView, Version 5, Dennington R, Keith T, Millam J (2009) Semichem Inc., Shawnee Mission, KS
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and non-covalent interactions. J Chem Phys 125:194101-1–194101-18
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, non-covalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functional and 12 other functionals. Theor Chem Acc 120:215–241
Perdew JP, Ruzsinszky A, Csonka GI, Constantin LA, Sun J (2009) Workhorse semilocal density functional for condensed matter physics and quantum chemistry. Phys Rev Lett 103:026403-1–026403-4
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colic–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Eichkorn K, Weigend F, Treutler O, Ahlrichs R (1997) Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor Chem Acc 97:119–124
Gutten O, Besseová I, Rulísek L (2011) Interaction of metal ions with biomolecular ligands: how accurate are calculated free energies associated with metal ion complexation? J Phys Chem 115:11394–11402
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comp Chem 24:669–681
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
NIST Computational Chemistry Comparison and Benchmark Database (2016) NIST standard reference database number 101 release 16a, August 2013. In: Russell D Johnson III (ed) (http://cccbdb.nist.gov/). Accessed 28 Apr 2016
Okuno Y (1997) Theoretical investigation of the mechanism of the Baeyer–Villiger reaction in nonpolar solvents. Chem Eur J 3:212
Benson SW (1982) The foundations of chemical kinetics. Krieger, Florida
Ardura D, Lopez R, Sordo TL (2005) Relative Gibbs energies in solution through continuum models: effect of the loss of translational degrees of freedom in bimolecular reactions on Gibbs energy barriers. J Phys Chem B 109:23618–23623
Alvarez-Idaboy JR, Reyes L, Cruz J (2006) A new specific mechanism for the acid catalysis of the addition step in the Baeyer–Villiger rearrangement. Org Lett 8:1763–1765
Galano A (2007) Influence of silicon defects on the adsorption of thiophene-like compounds on polycyclic aromatic hydrocarbons: a theoretical study using thiophene + coronae as the simplest model. J Phys Chem A 111:1677–1682
Matěj P, Jaroslav VB (2005) Theoretical description of copper Cu(I)/Cu(II) complexes in mixed ammine-aqua environment. DFT and ab initio quantum chemical study. Chem Phys 312:193–204
Sotomayor RG, Holguín AR, Romdhani A, Martinez F, Jouyban A (2013) Solution thermodynamics of piroxicam in some ethanol + water mixtures and correlation with the Jouyban–Acree model. J Solut Chem 42:358–371
Ivanova D, Deneva V, Nedeltcheva D, Kamounah FS, Gergov G, Hansen PE, Kawauchi S, Antonov L (2015) Tautomeric transformations of piroxicam in solution: a combined experimental and theoretical study. RSC Adv 5:31852–31860
Ferreira de Souza K, Martins JA, Pessine FBT, Custodio R (2010) A theoretical and spectroscopic study of conformational structures of piroxicam. Spectrochim Acta Part A 75:901–907
Allen LC (1975) A simple model of hydrogen bonding. J Am Chem Soc 97:6921–6940
Tita B, Rusu G, Tita D (2013) Thermal behaviour of active substance versus pharmaceutical compounds for Ibuprofen. Rev Chim (Bucharest) 64:472–476
Cini R, Pogni R, Basosi R, Donati A, Rossi C, Sabadini L, Rollo L, Lorenzini S, Gelli R, Marcolongo R (1995) Oxigen radical scavenger activity, EPR, NMR, molecular mechanism and extended-Hückel molecular orbital investigation of the bis(piroxicam)Cu(II) complex. Met Based Drugs 2:43–56
Méndez-Rojas MA, Cordova-Lozano F, Gojon-Zorrilla G, González-Vergara E, Quiroz MA (1999) Direct electrosynthesis of Cu, Cd, Zn complexes of piroxicam (4-hydroxy-2-methyl-N-(2-pyridyl) -2H-1,2-benzothiazine-3-carboxamide-1,1,-dioxide) and isoxicam (4-hydroxy-2-methyl-N-(5-methyl-3-isoxazolyl)-2H-1, 2-benzothiazine-3-carboxamide-1,1 dioxide) in non aqueous media by insitu generation of supporting electrolyte. Polyhedron 18:2651–2658
Gehad GM, El-Gamel NEA (2004) Synthesis, investigation and spectroscopic characterization of piroxicam ternary complexes of Fe(II), Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) with glycine and dl-phenylalanine. Spectrochim Acta Part A Mol Biomol Spect 60:3141–3154
Pasquarello A, Petri I, Salmon PS, Parisel O, Car R, Tóth E, Powell DH, Fischer HE, Helm L, Merbach AE (2001) First solvation shell of the Cu(II) aqua ion: evidence for fivefold coordination. Science 291:856–859
Bérces A, Nudaka T, Margl P, Ziegler T (1999) Solvation of Cu2+ in water and ammonia. Insight from static and dynamical density functional theory. J Phys Chem A 103:9693–9701
Salmon PS, Howells WS, Mills R (1987) The dynamics of water molecules in ionic solution. II. Quasi-elastic neutron scattering and tracer diffusion studies of the proton and ion dynamics in concentrated Ni2+, Cu2+ and Nd3+ aqueous solutions. J Phys C Solid State Phys 20:5727
El-Maali NA, Vire JC, Patriarche GJ, Ghandour MA (1989) Copper (II), lead (II) and cadmium (II) complexes with the antiinflammatory drugs piroxicam and tenoxicam. Anal Lett 22:3025–3039
Acknowledgments
L. G. L. -O acknowledges Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México, for the scholarship to pursue her major in Chemistry. This research was conducted under grants PAPIIT Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México IN222914 and PIAPI Facultad de Estudios Superiores Cuautitlán, Universidad Nacional Autónoma de México C23. Authors acknowledge Red Mexicana de Fisicoquímica Teórica (CONACyT) under grants 253498 and 271361, for supporting this investigation. We gratefully acknowledge the generous computing time provided by Dirección General de Cómputo y de Tecnologías de Información y Comunicación, Universidad Nacional Autónoma de México through the grants SC16-1-IR-100 and SC16-1-IR-112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “Festschrift in honour of A. Vela.”
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ledesma-Olvera, L.G., Agacino-Valdés, E. & Gómez-Balderas, R. Stability constants of Cu(II)-piroxicam complexes in solution: a DFT study. Theor Chem Acc 135, 241 (2016). https://doi.org/10.1007/s00214-016-1996-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1996-4