Abstract
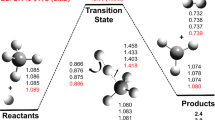
The potential energy surface for the first step of the methane oxidation CH4 + O2➔CH3 + HO2 was studied using the London-Eyring-Polanyi-Sato equation (LEPS) and the conventional transition-state theory (CTST). The calculated activation energy and rate constant values were in good agreement with the experimental and theoretical values reported in the literature using the shock tube technique and coupled cluster method respectively. The rate equation from CTST, although simple, provides good results to study the H-shift between methane and the oxygen molecules.









Similar content being viewed by others
References
Aul CJ, Metcalfe WK, Burke SM, Curran HJ, Petersen EL (2013) Ignition and kinetic modeling of methane and ethane fuel blends with oxygen: a design of experiments approach. Combust Flame 160:1153–1167. https://doi.org/10.1016/j.combustflame.2013.01.019
El Merhubi H, Kéromnès A, Catalano G, Lefort B, Le Moyne L (2016) A high pressure experimental and numerical study of methane ignition. Fuel 177:164–172. https://doi.org/10.1016/j.fuel.2016.03.016
Dale A, Lythall C, Aucott J, Sayer C (2002) High precision calorimetry to determine the enthalpy of combustion of methane. Thermochim Acta 382(1-2):47–54. https://doi.org/10.1016/S0040-6031(01)00735-3
Chenoweth K, Van Duin ACT, Goddard III WA (2008) ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J Phys Chem A 112:1040–1053. https://doi.org/10.1021/jp709896w
Page AJ, Moghtaderi B (2009) Molecular dynamics simulation of the low-temperature partial oxidation of CH4. J Phys Chem A 113(8):1539–1547. https://doi.org/10.1021/jp809576k
Srinivasan NK, Michael JV, Harding LB, Klippenstein SJ (2007) Experimental and theoretical rate constants for CH4+O2→CH3+HO2. Combust Flame 149:104–111. https://doi.org/10.1016/j.combustflame.2006.12.010
Rasmussen CL, Jakobsen JG, Glarborg P (2008) Experimental measurements and kinetic modeling of CH4/O2 and CH4/C2H6/O2conversion at high pressure. In J Chem Kinet 40(12):778–807. https://doi.org/10.1002/kin.20352
Mai TV-T, Duong MV, Le XT, Huynh LK, Ratkiewicz A (2014) Direct ab initio dynamics calculations of thermal rate constantsfor the CH4 + O2= CH3 + HO2 reaction. Struct Chem 25:1495–1503. https://doi.org/10.1007/s11224-014-0426-2
Giménez-López J, Millera A, Bilbao R, Alzueta MU (2015) Experimental and kinetic modeling study of the oxy-fuel oxidation of natural gas, CH4 and C2H6. Fuel 160:404–412. https://doi.org/10.1016/j.fuel.2015.07.087
Hashemi H, Christensen JM, Gersen S, Levinsky H, Klippenstein SJ, Glarborg P (2016) High-pressure oxidation of methane. Combust Flame 172:349–364. https://doi.org/10.1016/j.combustflame.2016.07.016
Ryu S-O, Shin KS, Hwang SM (2017) Determination of the rate coefficients of the CH4 + O2➔HO2 + CH3 and HCO + O2➔HO2 + CO reactions at high temperatures. Bull Kor Chem Soc 38:228–236. https://doi.org/10.1002/bkcs.11070
Skinner GB, Lifshitz A, Scheller K, Burcat A (1972) Kinetics of methane oxidation. J Chem Phys 56(8):3853–3861. https://doi.org/10.1063/1.1677790
Shaw R (1978) Semi-empirical extrapolation and estimation of rate constants for abstraction of H from methane by H, O, HO and O2. J Phys Chem Ref Data 7(3):1179–1190. https://doi.org/10.1063/1.555577
Tsang W, Hampson RF (1986) Chemical kinetic data vase for combustion chemistry. Part I. Methane and related compounds. J Phys Chem Ref Data 15(3):1087–1279. https://doi.org/10.1063/1.555759
Baulch DL, Cobos CJ, Cox RA, Esser C, Frank P, Just T, Kerr JA, Pilling MJ, Troe J, Walker RW, Warnatz J (1992) Evaluated kinetic data for combustion modeling. J Phys Chem Ref Data 21(3):411–734. https://doi.org/10.1063/1.555908
Baulch DL, Bowman CT, Cobos CJ, Cox RA, Just T, Kerr JA, Pilling MJ, Stocker D, Troe J, Tsang W, Walker RW, Warnatz J (2005) Evaluated kinetic data for combustion modeling: supplement II. J Phys Chem Ref Data 34:757–1397. https://doi.org/10.1063/1.1748524
Laidler KJ (1987) Chemical kinetics, 3rd edn. Harper Collins, New York
Galindo Hernández F, Méndez Ruiz F (2003) Determinación de la energía de activación para la reacción de H+H2 mediante el cálculo de superficies de energía potencial. Rev Mex Fis 49(3):264–270
Moss SJ, Coady CJ (1983) Potential-energy surfaces and transition-state theory. J Chem Educ 60(6):455–461. https://doi.org/10.1021/ed060p455
Sato S (1955) On a new method of drawing the potential energy surface. J Chem Phys 23:592–593. https://doi.org/10.1063/1.1742043
Wang X, Ben-Nun M, Levine RD (1995) Peripheral dynamics of the Cl + CH4 → HCl + CH3 reaction. Chem Phys 197:1–17. https://doi.org/10.1016/0301-0104(95)00134-A
Liu Y, Liu Z, Lv G, Jiang L, Sun J (2006) Product polarization distribution: Stereodynamics of the reactions Cl+CH4→HCl+CH3 and Cl+CD4→DCl+CD3. Chem Phys Lett 423:157–164. https://doi.org/10.1016/j.cplett.2006.03.059
Lemon WJ, Hase WL (1987) A potential energy function for the hydroperoxyl radical. J Phys Chem 91(6):1596–1602. https://doi.org/10.1021/j100290a061
Zhu R, Hsu C-C, Lin MC (2001) Ab initio study of the CH3 + O2 reaction: kinetics, mechanism and product branching probabilities. J Chem Phys 115(1):195–203. https://doi.org/10.1063/1.1376128
Ase P, Bock W, Snelson A (1986) Alkylperoxy and alkyl radicals. 1. Infrared spectra of CH3O2 and CH3O4CH3 and the ultraviolet photolysis of CH3O2 in argon+oxygen matrices. J Phys Chem 90(10):2099–2109. https://doi.org/10.1021/j100401a024
Knox JH (1971) Molecular partition functions. Wiley-Interscience, London
Kalman D (1982) Dot products, spherical coordinates, and 109°. Int J Math Educ Sci Technol 13(4):493–494. https://doi.org/10.1080/0020739820130412
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09 revision B.01. Gaussian Inc, Wallingford
MATLAB version 7.0.0. R14 (2004) The MathWorks Inc., Natick
Ree J, Kim YH, Shin HK (2007) Classical trajectory study of the formation of XeH and XeCl+ in the Xe++HCl collision. J Chem Phys 127(5):054304-1–054304-13. https://doi.org/10.1063/1.2751499
Acknowledgments
The authors would like to thank CONACYT-México for scholarship number 207214 and 18053 and 163234 CONACYT-México project grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection International Conference on Systems and Processes in Physics, Chemistry and Biology (ICSPPCB-2018) in honor of Professor Pratim K. Chattaraj on his sixtieth birthday
Electronic supplementary material
The Partition functions and kinetic constant values are included in the electronic supplementary material.
ESM 1
(DOCX 149 kb)
Rights and permissions
About this article
Cite this article
Aranda, C., Richaud, A., Méndez, F. et al. Theoretical rate constant of methane oxidation from the conventional transition-state theory. J Mol Model 24, 294 (2018). https://doi.org/10.1007/s00894-018-3829-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3829-y