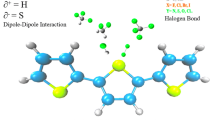
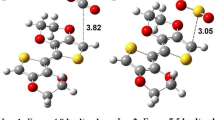
Abstract
An accurate comparison of the interaction of furan, pyrrole, and thiophene with different gaseous analytes is vital not only for understanding the sensing mechanism of corresponding polymers but also for rational design of new materials. In the present study, DFT calculations at (M05-2X/Aug-cc-PVDZ) have been performed to investigate the interaction behavior of furan, pyrrole, and thiophene (as models for their corresponding polymers) with different analytes (NH3, CO2, CO, N2H4, HCN, H2O2, H2S, CH4, CH3OH, SO2, SO3, H2O). The interaction of heterocycles with analytes is illustrated by changes in geometric, energetic, and electronic properties. SAPT calculations were performed for energy decomposition analysis to study the contribution of non-covalent components of the total interaction energy for each complex. Analysis of energetic and electronic properties reveals that all heterocycles are highly sensitive to SO3. The results suggest that sensing ability of polypyrrole is higher than polyfuran and polythiophene for all analytes.

SAPT0 energies (kcal mol-1) of furan, pyrrole, and thiophene with various gaseous analytesᅟ











Similar content being viewed by others
References
Harun MH, Saion E, Kassim A et al. (2007) Conjugated conducting polymers: a brief overview. Sensors Peterbrgh NH 2:63–68
Balint R, Cassidy NJ, Cartmell SH (2014) Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater 10:2341–2353
Lange U, Roznyatovskaya NV, Mirsky VM (2008) Conducting polymers in chemical sensors and arrays. Anal Chim Acta 614:1–26
Ak M, Yigitsoy B, Yagci Y, Toppare L (2007) Gas sensing property of a conducting copolymer. E-polymers 42
Patra A, Bendikov M (2010) Polyselenophenes. J Mater Chem 20:422–433
Ullah H, Shah A-HA, Bilal S, Ayub K (2014) Doping and dedoping processes of polypyrrole: DFT study with hybrid Functionals. J Phys Chem C 118:17819–17830
Kassim A, Basar ZB, Mahmud HNME (2002) Effects of preparation temperature on the conductivity of polypyrrole conducting polymer. J Chem Sci 114:155–162
Gomes AL, Casanovas J, Bertran O et al. (2011) Electronic properties of poly(thiophene-3-methyl acetate). J Polym Res 18:1509–1517
Zade SS, Bendikov M (2006) From Oligomers to polymer: convergence in the HOMO−LUMO gaps of conjugated Oligomers. Org Lett 8:5243–5246
Yoon H (2013) Current trends in sensors based on conducting polymer nanomaterials. Nano 3:524–549
Hutchison GR, Zhao Y-J, Delley B et al. (2003) Electronic structure of conducting polymers: limitations of oligomer extrapolation approximations and effects of heteroatoms. Phys Rev B 68:35204
Alemán C, Estrany F, Armelin E et al. (2007) A theoretical study on the interaction between N-methylpyrrole and 3,4-ethylenedioxythiophene units in copolymer molecules. Polymer (Guildf) 48:6162–6169
Rikukawa M, Sanui K (2000) Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog Polym Sci 25:1463–1502
Mahmud HNME, Kassim A, Zainal Z, Yunus WMM (2006) Fourier transform infrared study of polypyrrole–poly(vinyl alcohol) conducting polymer composite films: evidence of film formation and characterization. J Appl Polym Sci 100:4107–4113
Rad AS, Nasimi N, Jafari M et al. (2015) Ab-initio study of interaction of some atmospheric gases (SO2, NH3, H2O, CO, CH4 and CO2) with polypyrrole (3PPy) gas sensor: DFT calculations. Sensors Actuators B Chem 220:641–651
Hernandez SC, Chaudhuri D, Chen W et al. (2007) Single Polypyrrole Nanowire ammonia gas sensor. Electroanalysis 19:2125–2130
Rahman MA, Kumar P, Park D-S, Shim Y-B (2008) Electrochemical sensors based on organic conjugated polymers. Sensors 8:118–141
Rabias I, Howlin BJ (2001) A combined ab initio and semi-empirical study on the theoretical vibrational spectra and physical properties of polypyrrole. Comput Theor Polym Sci 11:241–249
Bai H, Shi G (2007) Gas sensors based on conducting polymers. Sensors 7:267–307
Ratcliffe NM (1990) Polypyrrole-based sensor for hydrazine and ammonia. Anal Chim Acta 239:257–262
Kumar Ram M, Yavuz O, Lahsangah V, Aldissi M (2005) CO gas sensing from ultrathin nano-composite conducting polymer film. Sensors Actuators B Chem 106:750–757
Timofeeva ON, Lubentsov BZ, Sudakova YZ et al. (1991) Conducting polymer interaction with gaseous substances. I. Water Synth Met 40:111–116
Cho J-H, Yu J-B, Kim J-S et al. (2005) Sensing behaviors of polypyrrole sensor under humidity condition. Sensors Actuators B Chem 108:389–392
Magaraphan R, Thuimthad A (2007) Polypyrrole-Organoclay Nanocomposites for gas sensors. NSTI-Nanotech 1:662–665
Hossein SH, Entezanti AA (1999) Polypyrrole based toxic gas sensors by mass and conductivity measurements. Iran Polym J 8:10261263199
Eslami M, Vahabi V, Ahmadi Peyghan A (2016) Sensing properties of BN nanotube toward carcinogenic 4-chloroaniline: a computational study. Phys E 76:6–11
Hadipour NL, Ahmadi Peyghan A, Soleymanabadi H (2015) Theoretical study on the al-doped ZnO Nanoclusters for CO chemical sensors. J Phys Chem C 119:6398–6404
Peyghan AA, Rastegar SF, Hadipour NL (2014) DFT study of NH3 adsorption on pristine, Ni- and Si-doped graphynes. Phys Lett A 378:2184–2190
Ullah H, Ayub K, Ullah Z et al. (2013) Theoretical insight of polypyrrole ammonia gas sensor. Synth Met 172:14–20
Ullah H, Shah A-HA, Bilal S, Ayub K (2013) DFT study of Polyaniline NH 3 , CO 2 , and CO gas sensors: comparison with recent experimental data. J Phys Chem C 117:23701–23711
Liu S-S, Bian L-J, Luan F et al. (2012) Theoretical study on polyaniline gas sensors: examinations of response mechanism for alcohol. Synth Met 162:862–867
Romanova J, Petrova J, Tadjer A, Gospodinova N (2010) Polyaniline–water interactions: a theoretical investigation with the polarisable continuum model. Synth Met 160:1050–1054
Bilal S, Bibi S, Ahmad SM, Shah A-HA (2015) Counterpoise-corrected energies, NBO, HOMO–LUMO and interaction energies of poly(o-aminophenol) for ammonia sensing by DFT methods. Synth Met 209:143–149
Frisch MJT, Schlegel GW, Scuseria HB, Robb GE, Cheeseman MA, Scalmani JR, Barone G, Mennucci V, Petersson B (2013) Gaussian 09 Rev. D.0.1. Gaussian Inc, Wallingford
Dennington, Roy; Keith, Todd; Millam J (2009) GaussView 5.0. Semichem Inc, Shawnee Mission, KS
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with Pparametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Turney JM, Simmonett AC, Parrish RM, Hohenstein EG, Evangelista F, Fermann JT, Mintz BJ, Burns LA, Wilke JJ, Abrams ML, Russ NJ, Leininger ML, Janssen CL, Seidl ET, Allen WD, Schaefer HF, King T (2011) PSI4: an open-source ab initio electronic structure program. WIREs Comput Mol Sci 2:556–565
Cybulski H, Sadlej J (2008) Symmetry-adapted perturbation-theory interaction-energy decomposition for hydrogen-bonded and stacking structures. J Chem Theory Comput 4:892–897
Su P, Li H (2009) Energy decomposition analysis of covalent bonds and intermolecular interactions. J Chem Phys 131:14102
Shokuhi Rad A, Zardoost MR, Abedini E (2015) First-principles study of terpyrrole as a potential hydrogen cyanide sensor: DFT calculations. J Mol Model 21:273
Rad AS, Valipour P, Gholizade A, Mousavinezhad SE (2015) Interaction of SO2 and SO3 on terthiophene (as a model of polythiophene gas sensor): DFT calculations. Chem Phys Lett 639:29–35
Shokuhi Rad A, Valipour P (2015) Interaction of methanol with some aniline and pyrrole derivatives: DFT calculations. Synth Met 209:502–511
Shokuhi Rad A (2015) Application of polythiophene to methanol vapor detection: an ab initio study. J Mol Model 21:285
Shokuhi Rad A, Esfahanian M, Ganjian E et al. (2016) The polythiophene molecular segment as a sensor model for H2O, HCN, NH3, SO3, and H2S: a density functional theory study. J Mol Model 22:127
Rad AS (2016) Terthiophene as a model sensor for some atmospheric gases: theoretical study. Mol Phys 114:584–591
Wasim F, Mahmood T, Ayub K (2016) An accurate cost effective DFT approach to study the sensing behaviour of polypyrrole towards nitrate ions in gas and aqueous phases. Phys Chem Chem Phys 18:19236–19247
Acknowledgments
This work is supported by the Higher Education Commission of Pakistan (Grants No. 1899, 2469 and 2981) and COMSATS Institute of Information Technology Abbottabad, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statements
The manuscript has not been submitted to more than one journal for simultaneous consideration.
Electronic supplementary material
ESM 1
(DOCX 3432 kb)
Rights and permissions
About this article
Cite this article
Sajid, H., Mahmood, T. & Ayub, K. An accurate comparative theoretical study of the interaction of furan, pyrrole, and thiophene with various gaseous analytes. J Mol Model 23, 295 (2017). https://doi.org/10.1007/s00894-017-3458-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3458-x