Abstract
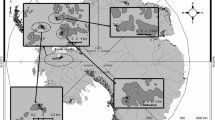
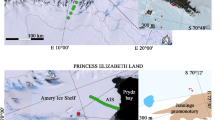
The aim of the present study was to investigate the taxonomic identity of yeasts isolated from the Antarctic continent and to evaluate their ability to produce enzymes (lipase, protease and xylanase) at low and moderate temperatures. A total of 97 yeast strains were recovered from marine and terrestrial samples collected in the Antarctica. The highest amount of yeast strains was obtained from marine sediments, followed by lichens, ornithogenic soils, sea stars, Salpa sp., algae, sea urchin, sea squirt, stone with lichens, Nacella concinna, sea sponge, sea isopod and sea snail. Data from polyphasic taxonomy revealed the presence of 21 yeast species, distributed in the phylum Ascomycota (n = 8) and Basidiomycota (n = 13). Representatives of encapsulated yeasts, belonging to genera Rhodotorula and Cryptococcus were recovered from 7 different Antarctic samples. Moreover, Candida glaebosa, Cryptococcus victoriae, Meyerozyma (Pichia) guilliermondii, Rhodotorula mucilaginosa and R. laryngis were the most abundant yeast species recovered. This is the first report of the occurrence of some species of yeasts recovered from Antarctic marine invertebrates. Additionally, results from enzymes production at low/moderate temperatures revealed that the Antarctic environment contains metabolically diverse cultivable yeasts, which could be considered as a target for biotechnological applications. Among the evaluated yeasts in the present study 46.39, 37.11 and 14.43 % were able to produce lipase (at 15 °C), xylanase (at 15 °C) and protease (at 25 °C), respectively. The majority of lipolytic, proteolytic and xylanolytic strains were distributed in the phylum Basidiomycota and were mainly recovered from sea stars, lichens, sea urchin and marine sediments.




Similar content being viewed by others
References
Almeida JMGCF (2005) Yeast community survey in the Tagus estuary. FEMS Microbiol Ecol 53:295–303
Arenz BE, Blanchette RA (2010) Distribution and abundance of soil fungi in Antarctica at sites on the Peninsula, Ross Sea Region and McMurdo Dry Valleys. Soil Biol Biochem 43:308–315
Bölter M, Kandeler E, Pietr SJ, Seppelt RD (2002) Heterotrophic Microbes, Microbial and Enzymatic Activity in Antarctic Soils. Geoecol Antarct Ice-Free Coast Landsc Ecol Stud 154:189–214
Bon EP, Costa RB, Silva MVA, Leitao VSF, Freitas SP, Ferrara MA (2008) Mercado e Perspectivas de Uso de Enzimas Industriais e Especiais no Brasil. In: Enzimas em Biotecnologia- Produção, Aplicações e Mercado, Ed. Interciência, Rio de Janeiro 20:463–488
Burgaud G, Arzur D, Durand L, Cambon-Bonavita M, Barbier G (2010) Marine culturable yeasts in deep-sea hydrothermal vents: species richness and association with fauna. FEMS Microbiol Ecol 73:121–133
Carrasco M, Rozas JM, Barahona S, Alcaíno J, Cifuentes V, Baeza M (2012) Diversity and extracellular enzymatic activities of yeasts isolated from King George Island, the sub-Antarctic region. BMC Microbiol 12:251
Charoenchai C, Fleet GH, Henschke PA, Todd B (1997) Screening of non-Saccharomyces wine yeasts for the presence of extracellular hydrolytic enzymes. Aust J Grape Wine Res 3:2–8
Connell L, Redman R, Craig S, Scorzetti G, Iszard M, Rodriguez R (2008) Diversity of soil yeasts isolated from South Victoria Land, Antarctica. Microbiol Ecol 56:448–459
Crowe JH, Crowe LM, Carpenter JF, Wistrom CA (1987) Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J 242:1–10
Crowe JH, Hoekstra FA, Crowe LM (1992) Anhydrobiosis. Annu Rev Physiol 54:579–599
Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1:200–208
Frisvad JC (2008) Fungi in cold ecosystems. In: Margesin R, Schinner F, Marx JC, Gerday C (eds) Psychrophiles: from biodiversity to biotechnology. Springer, Berlin, pp 137–156
Gadanho M, Sampaio JP (2009) Cryptococcus ibericus sp. nov., Cryptococcus aciditolerans sp. nov. and Cryptococcus metallitolerans sp. nov., a new ecoclade of anamorphic basidiomycetous yeast species from an extreme environment associated with acid rock drainage in São Domingos pyrite mine, Portugal. Int J Syst Evol Microbiol 59:2375–2379
Geok LP, Razak CAN, Rahman RNZA, Basri M, Salleh AB (2003) Isolation and screening of an extracellular organic solvent-tolerant protease producer. Biochem Eng J 13:73–77
Gomes J, Gomes I, Steiner W (2000) Thermolabile xylanase of the Antarctic yeast Cryptococcus adeliae: production and properties. Extremophiles 4:227–235
Hall TA (1999) Bio Edit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/ 98/NT. Nucl Acids Symp Ser 41:95–98
Joseph B, Ramteke PW, Thomas G (2008) Cold active microbial lipases: some hot issues and recent developments. Biotechnol Adv 26:457–470
Knob A, Carmona EC (2008) Xylanase production by Penicillium sclerotiorum and its characterization. World Appl Sci J 4(2):277–283
Kohlmeyer J, Kohlmeyer E (1979) Marine micology: the higher fungi. Academic Press, New York
Kouker G, Jaeger KE (1987) Specific and sensitive plate assay for bacterial lipases. Appl Environ Microbiol 53:211–213
Kurtzman CP, Fell JW, Boekhout T, Robert V (2011) Methods for isolation, phenotypic characterization and maintenance of yeasts. In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts: a taxonomic study, 5th edn. Elsevier, San Diego, pp 88–110
Kutty SN, Philip R (2008) Marine yeasts—a review. Yeast 25:465–483
Li J, Chi Z, Wang X, Peng Y, Chi Z (2009) The selection of alkaline protease-producing yeasts from marine environments and evaluation of their bioactive peptide production. Chin J Oceanol Limnol 27:753–761
Margesin R (2000) Potential of cold-adapted microorganisms for bioremediation of oil-polluted Alpine soils. Int Biodeterior Biodegrad 46:3–10
Margesin R, Miteva V (2011) Diversity and ecology of psychrophilic microorganisms. Res Microbiol 162:346–361
Margesin R, Schinner F, Marx JC, Gerday C (eds) (2008) Psychrophiles: from bodiversity to biotechnology. Springer, Berlin, Germany, pp 352–360
Maria PD, Carboni-Oerlemans C, Tuin B, Bargeman G, Meer A, Gemert R (2005) Biotechnological applications of Candida antarctica lipase A: state-of-the-art. J Mol Catal B Enzym 37:36–46
Miller GL (1959) Use of dinitrosalicylic acid reagent for the production of reducing sugars. Anal Chem 31:426–620
Ohta K, Fujimoto H, Fujii S, Wakiyama M (2010) Cell-associated β-xylosidase from Aureobasidium pullulans ATCC 20524: purification, properties, and characterization of the encoding gene. J Biosci Bioeng 110:152–157
Onofri S, Selbmann L, Hoog GS, Grube M, Barreca D, Ruisi S, Zucconi L (2007) Evolution and adaptation of fungi at boundaries of life. Adv Space Res 40:1657–1664
Petrescu I, Lamotte-Braaseur J, Chessa JP, Ntarima P, Claeyssens M, Devreese B, Marino G, Gerday C (2000) Xylanase from the psychrophilic yeast Cryptococcus adeliae. Extremophiles 4:137–144
Pointing SB (1999) Qualitative methods for the determination of lignocellulolytic enzyme production by tropical fungi. Fungal Divers 2:17–33
Quanfu W, Yanhua H, Yu D, Peisheng Y (2012) Purification and biochemical characterization of a cold-active lipase from Antarctic sea ice bacteria Pseudoalteromonas sp. NJ 70. Mol Biol Rep 39:9233–9238
Rao S, Mizutani O, Hirano T, Masaki K, Iefuji H (2011) Purification and characterization of a novel aspartic protease from basidiomycetous yeast Cryptococcus sp. S-2. J Biosci Bioeng 112:441–446
Ray MK, Devi KU, Kumar GS, Shivaji S (1992) Extracellular Protease from the Antarctic Yeast Candida humicola. Appl Environ Microbiol 58:1918–1923
Richards TA, Jones MDM, Leonard G, Bass D (2012) Marine fungi: their ecology and molecular diversity. Ann Rev Mar Sci 4:495–522
Robinson CH (2001) Cold adaptation in Arctic and Antarctic fungi. New Phytol 151:341–353
Ruisi S, Barreca D, Selbmann L, Zucconi L, Onofri S (2007) Fungi in Antarctica. Rev Environ Sci Bio/Technol 6:127–141
Russell NJ (1990) Cold adaptation of microorganisms. Philos Trans R Soc Lond Ser B 326:595–611
Russell NJ, Cowan DA (2006) Handling of psychrophilic microorganisms. In: Rainey FA, Oren A (eds) Methods in microbiology, extremophiles, vol 35. Academic Press, London, pp 371–393
Shivaji S, Prasad GS (2009) Antarctic yeasts: biodiversity and potential applications. In: Satyanarayana T, Kunze G (eds) Yeast biotechnology: diversity and applications. Springer, Amsterdam, pp 3–18
Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 75:403–433
Subramaniyan S, Prema P (2002) Biotechnology of microbial xylanases: enzymology, molecular biology and application. Crit Rev Biotechnol 22:33–46
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA 4: molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Higgins DG, Gibson TJ, Clustal W (1994) Improving the sensitivity of progressive multiple alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Turchetti B, Buzzini P, Goretti M, Branda E, Diolaiuti G, D’Agata C, Smiraglia C, Vaughan-Martini A (2008) Psychrophilic yeasts in glacial environments of Alpine glaciers. FEMS Microbiol Ecol 63:73–83
Turkiewicz M, Pazgier M, Kalinowska H, Bielecki S (2003) A cold-adapted extracellular serine proteinase of the yeast Leucosporidium antarcticum. Extremophiles 7:435–442
Uetake J, Yoshitaka Y, Naoko N, Hiroshi K (2012) Isolation of oligotrophic yeasts from supraglacial environments of different altitude on the Gulkana Glacier (Alaska). FEMS Microbiol Ecol 82:279–286
Vakhlu J, Kour A (2006) Yeast lipases: enzyme purification, biochemical properties and gene cloning. Electron J Biotechnol 9:69–85
Vaz ABM, Rosa LH, Vieira ML, Garcia V, Brandão LR, Teixeira LCRS, Moliné M, Libkind D, Van BMG, Rosa CA (2011) The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz J Microbiol 43:937–947
Vermelho AB, Melo ACN, Sá MHB, Santos ALS, Davila-Levy CM, Couri S, Bon E (2008) Enzimas Proteolíticas: aplicações Biotecnológicas. In: Enzimas em Biotecnologia- Produção, Aplicações e Mercado, Ed. Interciência, Rio de Janeiro 11:273–287
Vishniac HS (2006) Yeast biodiversity in the Antarctic. In: Rosa CA, Péter G (eds) Biodiversity and ecophysiology of yeasts. Springer, Berlin, pp 221–240
Vogel HJ (1956) A convenient growth medium for Neurospora crassa. Microb Genetics Bull 13:42–43
Yang J, Koga Y, Nakano H, Yamane T (2002) Modifying the chain-length selectivity of the lipase from Burkholderia cepacia KWI-56 through in vitro combinatorial mutagenesis in the substrate-binding site. Protein Eng 15:147–152
Yergeau E, Kowalchuk GA (2008) Responses of Antarctic soil microbial communities and associated functions to temperature and freeze–thaw cycle frequency. Environ Microbiol 10:2223–2235
Acknowledgments
The authors would like to thank Prof. Vivian Helena Pellizari, Prof. Itamar Soares Melo and Prof. Eduardo Carlos Hadju for their contribution in the collection of marine and terrestrial Antarctic samples, as well as Raymond J. Burgess for the English Review. A. Duarte was supported by Ph.D. grants from CAPES and FAPESP (2010/08352-5). Part of the research was supported by the European Community’s Seventh Framework Program (FP7 2010–2013, BAMMBO Project). L.D. Sette thanks FAPESP for the financial support (FAPESP grant 2010/17033-0) and PROANTAR Program for the sampling support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. da Costa.
Rights and permissions
About this article
Cite this article
Duarte, A.W.F., Dayo-Owoyemi, I., Nobre, F.S. et al. Taxonomic assessment and enzymes production by yeasts isolated from marine and terrestrial Antarctic samples. Extremophiles 17, 1023–1035 (2013). https://doi.org/10.1007/s00792-013-0584-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0584-y