Abstract
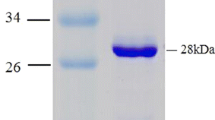
Enzymes produced by halophilic archaea are generally heat resistant and organic solvent tolerant, and accordingly important for biocatalytic applications in ‘green chemistry’, frequently requiring a low-water environment. NAD+-dependent glutamate dehydrogenase from an extremely halophilic archaeon Halobacterium salinarum strain NRC-36014 was selected to explore the biotechnological potential of this enzyme and genetically engineered derivatives. Over-expression in a halophilic host Haloferax volcanii provided a soluble, active recombinant enzyme, not achievable in mesophilic Escherichia coli, and an efficient purification procedure was developed. pH and salt dependence, thermostability, organic solvent stability and kinetic parameters were explored. The enzyme is active up to 90 °C and fully stable up to 70 °C. It shows good tolerance of various miscible organic solvents. High concentrations of salt may be substituted with 30 % DMSO or betaine with good stability and activity. The robustness of this enzyme under a wide range of conditions offers a promising scaffold for protein engineering.








Similar content being viewed by others
References
Aghajanian S, Engel PC (1997) Re-activation of Clostridium symbiosum glutamate dehydrogenase from subunits denatured by urea. Biochem J 326:649–655
Adams MWW, Perler FB, Kelly RM (1995) Extremozymes: expanding the limits of biocatalysis. Biotechnology 13:662–668
Allers T, Barak S, Liddell S, Wardell K, Mevarech M (2010) Improved strains and plasmid vectors for conditional overexpression of His-tagged proteins in Haloferax volcanii. Appl Environ Microbiol 76:1759–1769
Allers T, Ngo HP, Mevarech M, Lloyd RG (2004) Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based in the leuB and trpA genes. Appl Environ Microbiol 70:943–953
Benachenhou N, Baldacci G (1991) The gene for a halophilic glutamate dehydrogenase: sequence, transcription analysis and phylogenetic implications. Mol Gen Genet 230:345–352
Bolivar JM, Cava F, Mateo C, Rocha-Martín J, Guisán JM, Berenguer J, Fernandez-Lafuente R (2008) Immobilization-stabilization of a new recombinant glutamate dehydrogenase from Thermus thermophilus. Appl Microbiol Biotechnol 80:49–58
Bonete MJ, Camacho ML, Cadenas E (1986) Purification and some properties of NAD+-dependent glutamate dehydrogenase from Halobacterium halobium. Int J Biochem 18:785–789
Bonete MJ, Camacho ML, Cadenas E (1987) A new glutamate dehydrogenase from Halobacterium halobium with different coenzyme specificity. Int J Biochem 19:1149–1155
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Busca P, Paradisi F, Moynihan E, Maguire AR, Engel PC (2004) Enantioselective synthesis of non-natural amino acids using phenylalanine dehydrogenases modified by site-directed mutagenesis. Org Biomol Chem 2:2684–2691
Carrigan JB, Coughlan S, Engel PC (2005) Properties of thermostable glutamate dehydrogenase of the mesophilic anaerobe Peptostreptococcus asaccharolyticus purified by a novel method after over-expression in a Escherichia coli host. FEMS Microbiol Lett 2144:53–59
Cline SW, Lam WL, Charlebois RL, Schalkwyk LC, Doolittle WF (1989) Transformation methods for halophilic archaebacteria. Can J Microbiol 35:148–152
Cline SW, Pfeifer F, Doolittle WF (1995) Transformation of halophilic Archaea. In: Robb FT, Sowers KR, Place AR, Schreier HJ (eds) Archaea: a laboratory manual. CSHL Press, New York, pp 197–204
Connaris H, Chaudhuri JB, Danson MJ, Hough DW (1999) Expression, reactivation, and purification of enzymes from Haloferax volcanii in Escherichia coli. Biotechnol Bioeng 64:38–45
De Champdoré M, Staiano M, Rossi M, D’Auria S (2007) Proteins from extremophiles as stable tools for advanced biotechnological applications of high social interest. J R Soc Interface 4:183–191
Diaz S, Perez-Pomares F, Pire C, Ferrer J, Bonete MJ (2006) Gene cloning, heterologous overexpression and optimized refolding of NAD-glutamate dehydrogenase from Haloferax mediterranei. Extremophiles 10:105–115
Dyall-Smith M (2006) The Halohand book. Protocols for halobacterial genetics. Version 6 http://www.microbiol.unimelb.edu.au/people/dyallsmith/resources/halohandbook/HaloHandbook_v6_06.pdf
Engel PC, Paradisi F (2010) Novel enzymes for biotransformation and resolution of amino acids. In: Vederas JC (ed) Comprehensive natural products chemistry II chemistry and biology, vol 5. Elsevier, Oxford, 71–90
Ferrer J, Pérez-Pomares F, Bonete MJ (1996) NADP-glutamate dehydrogenase from the halophilic archaeon Haloferax mediterraner. enzyme purification, N-terminal sequence and stability. FEMS Microbiol Lett 141:59–63
Ferrer J, Pire C, Bonete MJ (2001) Kinetic behavior of NADP-glutamate dehydrogenase from an extreme halophile Haloferax mediterranei in halophilic conditions (3 M KCl) and in glycerol. Biocatal Biotransform 19:235–249
Gupta MN, Batra R, Tyagi R, Sharma A (1997) Polarity Index: the guiding solvent parameter for enzyme stability in aqueous-organic cosolvent mixtures. Biotechnol Prog 13:284–288
Hartman LM, Norais C, Badger JH, Delmas S, Haldenby S, Madupu R, Robinson J, Khouri H, Ren Q, Lowe TM, Maupin-Furlow J, Pohlschroder M, Daniels C, Pfeiffer F, Allers T, Eisen JA (2010) The complete genome sequence of Haloferax volcanii DS2, a model archaeon. PLoS One 5:e9605
Hayden MB, Bonete MJ, Brown PE, Moir AJG, Engel PC (2002) Glutamate dehydrogenase of Halobacterium salinarum: evidence that the gene sequence currently assigned to the NADP+-dependent enzyme is in fact that of the NAD+-dependent glutamate dehydrogenase. FEMS Microbiol Lett 211:37–41
Holmes M, Pfeifer F, Dyall-Smith ML (1994) Improved shuttle vectors for Haloferax volcanii including a dual-resistance plasmid. Gene 146:117–121
Holmes ML, Dyall-Smith ML (1990) A plasmid vector with a selectable marker for halophilic archaebacteria. J Bacteriol 172:756–761
Hudson RC, Daniel RM (1993) l-glutamate dehydrogenases: distribution properties and mechanism. Comp Biochem Physiol B 106:767–792
Ibrahim I (2010) The four glutamate dehydrogenases of the halophilic archaeon Halobacterium salinarum strain NRC-36014 and NRC-1. PhD thesis, University College Dublin, Republic of Ireland
Ingoldsby LM (2005) The glutamate dehydrogenases of the halophilic archaeon Halobacterium salinarum. PhD thesis, University College Dublin, Republic of Ireland
Ingoldsby LM, Geoghegan KF, Hayden BM, Engel PC (2005) The discovery of four distinct glutamate dehydrogenase genes in a strain of Halobacterium salinarum. Gene 349:237–244
Jolley KA, Rapaport E, Hough DW, Danson MJ, Woods WG, Dyall-Smith ML (1996) Dihydrolipoamide dehydrogenase from the halophilic archaeon Haloferax volcanii: homologous overexpression of the cloned gene. J Bacteriol 178:3044–3048
Kim J, Dordick JS (1997) Unusual salt and solvent dependence of a protease from an extreme halophile. Biotechnol Bioeng 55:471–479
Large A, Stamme C, Lange C, Duan ZH, Allers T, Soppa J, Lund PA (2007) Characterization of a tightly controlled promoter of the halophilic archaeon Haloferax volcanii and its use in the analysis of the essential cct1 gene. Mol Microbiol 66:1092–1106
Matsuo T, Ikeda A, Seki H, Ichimata T, Sugimori D, Nakamura S (2001) Cloning and expression of the ferredoxin gene from extremely halophilic archaeon Haloarcula japonica strain TR-1. Biometals 14:135–142
Ng WV, Kennedy SP, Mahairas GG, Berquist B, Pan M, Shukla HD, Lasky SR, Baliga NS, Thorsson V, Sbrogna J, Swartzell S, Weir D, Hall J, Dahl TA, Welti R, Goo YA, Leithauser B, Keller K, Cruz R, Danson MJ, Hough DW, Maddocks DG, Jablonski PE, Krebs MP, Angevine CM, Dale H, Isenbarger TA, Peck RF, Pohlschroder M, Spudich JL, Jung KW, Alam M, Freitas T, Hou S, Daniels CJ, Dennis PP, Omer AD, Ebhardt H, Lowe TM, Liang P, Riley M, Hood L, DasSarma S (2000) Genome sequence of Halobacterium species NRC-1. Proc Natl Acad Sci USA 22:12176–12181
Paradisi F, Collins S, Maguire AR, Engel PC (2007) Phenylalanine dehydrogenase mutants: efficient catalysts for the synthesis of non-natural phenylalanine derivatives. J Biotechnol 128:408–411
Perez-Pomares F, Bautista V, Ferrer J, Pire C, Marhuenda-Egea FC, Bonete MJ (2003) Alpha-amylase activity from the halophilic archaeon Haloferax mediterranei. Extremophiles 7:299–306
Pire C, Esclapez J, Ferrer J, Bonete MJ (2001) Heterologous expression of glucose dehydrogenase from the halophilic archaeon Haloferax mediterranei, an enzyme of the medium chain dehydrogenase family. FEMS Microbiol Lett 200:221–227
Reslow M, Adlercreutz P, Mattiasson B (1992) Modification of the microenvironment of enzymes in organic solvents. Substitution of water by polar solvents. Biocatal Biotransform 6:307–318
Rinaldi R, Pompa PP, Maruccio G, Biasco A, Visconti P, Pisignano D, Blasi L, Sgarbi N, Krebs B, Cingolani R (2004) Self-assembling of proteins and enzymes at nanoscale for biodevice applications. Nanobiotechnology 151:101–108
Robinson JL, Pyzyna B, Atrasz RG, Henderson CA, Morrill KL, Burd AM, Desoucy E, Fogleman RE 3rd, Naylor JB, Steele SM, Elliott DR, Leyva KJ, Shand RF (2005) Growth kinetics of extremely halophilic archaea (family halobacteriaceae) as revealed by Arrhenius plots. J Bacteriol 187:923–929
Ruiz MD, De Castro ER (1997) Effects of organic solvents on the activity and stability of an extracellular protease secreted by the haloalkaliphilic archaeon Natrialba magadii. J Ind Microbiol Biotechnol 34:111–115
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Plainview, pp 49–55
Seah SYK, Britton KL, Rice DW, Asano Y, Engel PC (2002) Single amino acid-substitution in Bacillus sphaericus phenylalanine dehydrogenase dramatically increases its discrimination between phenylalanine and tyrosine substrates. Biochemistry 41:11390–11397
Sharkey MA, Engel PC (2009) Modular coenzyme specificity: a domain-swopped chimera of glutamate dehydrogenase. Proteins 77:268–278
Singh SM, Panda AK (2005) Solubilization and refolding of bacterial inclusion body proteins. J Biosci Bioeng 4:303–310
Stepanov VM, Rudenskaya GN, Revina LP, Gryaznova YB, Lysogorskaya EN, Filippova IY, Ivanova II (1992) A serine protease of an archaebacterium Halobacterium mediterranei. A homologue of eubacterial subtilisin. Biochem J 285:281–286
Tokunaga H, Arakawa T, Tokunaga M (2008) Engineering of halophilic enzymes: two acidic amino acid residues at the carboxy-terminal region confer halophilic characteristics to Halomonas and Pseudomonas nucleoside diphosphate kinases. Protein Sci 17:1603–1610
Tsumoto K, Ejima D, Kumagai I, Arakawa T (2003) Practical considerations in refolding proteins from inclusion bodies. Protein Expr Pur 28:1–8
Van den Burg B (2003) Extremophiles as a source for novel enzymes. Curr Opin Microbiol 6:213–218
Wang XG, Britton KL, Stillman TJ, Rice DW, Engel PC (2001) Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis. Eur J Biochem 268:5791–5799
Wilkinson GN (1961) Statistical estimations in enzyme kinetics. Biochem J 80:324–332
Acknowledgments
N. M. was supported by an Ad Astra scholarship from University College Dublin. We are also grateful to Dr. Peter Lund and Dr. Andrew Large (University of Birmingham) for providing us the halophilic expression vector and host strain and to Science Foundation Ireland for a Research Fellowship (2006–2011) to P.C.E.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Robb.
Rights and permissions
About this article
Cite this article
Munawar, N., Engel, P.C. Overexpression in a non-native halophilic host and biotechnological potential of NAD+-dependent glutamate dehydrogenase from Halobacterium salinarum strain NRC-36014. Extremophiles 16, 463–476 (2012). https://doi.org/10.1007/s00792-012-0446-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-012-0446-z