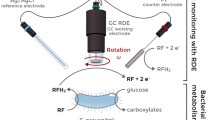
Abstract
Changes in the redox metabolism in the anaerobic, extremely thermophilic, hydrogen-forming bacterium Caldicellulosiruptor saccharolyticus were probed for the first time in vivo using mediated amperometry with ferricyanide as a thermotolerant external mediator. Clear differences in the intracellular electron flow were observed when cells were supplied with different carbon sources. A higher electrochemical response was detected when cells were supplied with xylose than with sucrose or glucose. Moreover, using the mediated electrochemical method, it was possible to detect differences in the electron flow between cells harvested in the exponential and stationary growth phases. The electron flow of C. saccharolyticus was dependent on the NADH- and reduced ferredoxin generation flux and the competitive behavior of cytosolic and membrane-associated oxidoreductases. Sodium oxamate was used to inhibit the NADH-dependent lactate dehydrogenase, upon which more NADH was directed to membrane-associated enzymes for ferricyanide reduction, leading to a higher electrochemical signal. The method is noninvasive and the results presented here demonstrate that this method can be used to accurately detect changes in the intracellular electron flow and to probe redox enzyme properties of a strictly anaerobic thermophile in vivo.






Similar content being viewed by others
References
Almeida JRM, Roder A, Modig T, Laadan B, Liden G, Gorwa-Grauslund MF (2008) NADH- vs NADPH-coupled reduction of 5-hydroxymethyl furfural (HMF) and its implications on product distribution in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 78(6):939–945
Angenent LT, Karim K, Al-Dahhan MH, Domiguez-Espinosa R (2004) Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol 22(9):477–485
Arrigo AP (1999) Gene expression and the thiol redox state. Free Radic Biol Med 27(9–10):936–944
Bielen AAM, Willquist K, Engman J, van der Oost J, van Niel EWJ, Kengen SWM (2010) Pyrophosphate as a central energy carrier in the hydrogen-producing extremely thermophilic Caldicellulosiruptor saccharolyticus. FEMS Microbiol Lett 307(1):48–54
Bloomfield V, Alberty RA (1963) Abortive complexes in dehydrogenase reactions. J Biol Chem 238(8):2817–2822
Brown DM, Upcroft JA, Edwards MR, Upcroft P (1998) Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int J Parasitol 28(1):149–164
Buchanan BB, Schurmann P, Wolosiuk RA, Jacquot JP (2002) The ferredoxin/thioredoxin system: from discovery to molecular structures and beyond. Photosynth Res 73(1–3):215–222
Catterall K, Morris K, Gladman C, Zhao HJ, Pasco N, John R (2001) The use of microorganisms with broad range substrate utilisation for the ferricyanide-mediated rapid determination of biochemical oxygen demand. Talanta 55(6):1187–1194
Chaubey A, Malhotra BD (2002) Mediated biosensors. Biosens Bioelectron 17(6–7):441–456
de Vrije T, Mars AE, Budde MA, Lai MH, Dijkema C, de Waard P, Claassen PAM (2007) Glycolytic pathway and hydrogen yield studies of the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl Microbiol Biotechnol 74(6):1358–1367
de Vrije T, Bakker RR, Budde MAW, Lai MH, Mars AE, Claassen PAM (2009) Efficient hydrogen production from the lignocellulosic energy crop Miscanthus by the extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol Biofuels 2(12):12
Gao H, Zhao MH, Zhang XL, Jin WR (2006) Measurement of enzyme activity in single cells by voltammetry using a microcell with a positionable dual electrode. Anal Chem 78(1):231–238
Harwood GW, Pouton CW (1996) Amperometric enzyme biosensors for the analysis of drugs and metabolites. Adv Drug Deliv Rev 18(2):163–191
Heiskanen A, Spégel C, Kostesha N, Lindahl S, Ruzgas T, Emneus J (2009) Mediator-assisted simultaneous probing of cytosolic and mitochondrial redox activity in living cells. Anal Biochem 384(1):11–19
Ikeda T (2004) A novel electrochemical approach to the characterization of oxidoreductase reactions. Chem Record 4(3):192–203
Ikeda T, Kano K (2001) An electrochemical approach to the studies of biological redox reactions and their applications to biosensors, bioreactors, and biofuel cells. J Biosci Bioeng 92(1):9–18
Kim I, Yun H, Iwahashi H, Jin I (2006) Genome-wide expression analyses of adaptive response against medadione-induced oxidative stress in Saccharomyces cerevisiae KNU5377. Process Biochem 41(11):2305–2313
Kolthoff IM, Lingane JJ (1952) Polarography. Interscience Publishers, New York
Kondo T, Ikeda T (1999) An electrochemical method for the measurements of substrate-oxidizing activity of acetic acid bacteria using a carbon-paste electrode modified with immobilized bacteria. Appl Microbiol Biotechnol 51(5):664–668
Kostesha N, Heiskanen A, Spegel C, Hahn-Hagerdal B, Gorwa-Grauslund MF, Emneus J (2009a) Real-time detection of cofactor availability in genetically modified living Saccharomyces cerevisiae cells—simultaneous probing of different geno- and phenotypes. Bioelectrochemistry 76(1–2):180–188
Kostesha NV, Almeida JRM, Heiskanen AR, Gorwa-Grauslund MF, Hahn-Hagerdal B, Emneus J (2009b) Electrochemical probing of in vivo 5-hydroxymethyl furfural reduction in Saccharomyces cerevisiae. Anal Chem 81(24):9896–9901
Kraemer JT, Bagley DM (2007) Improving the yield from fermentative hydrogen production. Biotechnol Lett 29(5):685–695
Krylov SN, Zhang ZR, Chan NWC, Arriaga E, Palcic MM, Dovichi NJ (1999) Correlating cell cycle with metabolism in single cells: combination of image and metabolic cytometry. Cytometry 37(1):14–20
Mashego MR, Rumbold K, De Mey M, Vandamme E, Soetaert W, Heijnen JJ (2007) Microbial metabolomics: past, present and future methodologies. Biotechnol Lett 29(1):1–16
Nakamura H, Nakamura K, Yodoi J (1997) Redox regulation of cellular activation. Annu Rev Immunol 15:351–369
Powis G, Briehl M, Oblong J (1995) Redox signaling and the control of cell-growth and death. Pharmacol Ther 68(1):149–173
Rainey FA, Donnison AM, Janssen PH, Saul D, Rodrigo A, Bergquist PL, Danie RM, Stackebrandt E, Morgan HW (1994) Description of Caldicellulosiruptor saccharolyticus gen-nov, sp, nov. an obligately anaerobic, extremely thermophilic, cellulolytic bacterium. FEMS Microbiol Lett 120(3):263–266
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30(11):1191–1212
Shaw AJ, Jenney E, Adams MWW, Lynd LR (2008) End-product pathways in the xylose fermenting bacterium Thermoanaerobacterium saccharolyticum. Enzyme Microb Technol 42(6):453–458
Spegel CF, Heiskanen AR, Kostesha N, Johanson TH, Gorwa-Grauslund MF, Koudelka-Hep M, Emneus J, Ruzgas T (2007) Amperometric response from the glycolytic versus the pentose phosphate pathway in Saccharomyces cerevisiae cells. Anal Chem 79(23):8919–8926
Takayama K, Kurosaki T, Ikeda T (1993) Mediated electrocatalysis at a biocatalyst electrode based on a bacterium, Gluconobacter industrius. J Electroanal Chem 356(1–2):295–301
van de Werken HJG, Verhaart MRA, van Fossen AL, Willquist K, Lewis DL, Nichols JD, Goorissen HP, Mongodin EF, Nelson KE, van Niel EWJ, Stams AJM, Ward DE, de Vos WM, van der Oost J, Kelly RM, Kengen SWM (2008) Hydrogenomics of the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl Environ Microbiol 74(21):6720–6729
van Fossen AL, Lewis DL, Nichols JD, Kelly RM (2008) Polysaccharide degradation and synthesis by extremely nermophilic anaerobes. Incredible anaerobes: from physiology to genomics to fuels. Ann Acad Sci, New York 1125:322–337
van Fossen AL, Verhaart MR, Kengen SM, Kelly RM (2009) Carbohydrate utilization patterns for the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus reveal broad growth substrate preferences. Appl Environ Microbiol 75:7718–7724
van Niel EWJ, Claassen PAM, Stams AMJ (2003) Substrate and product inhibition of hydrogen production by the extreme thermophile, Caldicellulosiruptor saccharolyticus. Biotechnol Bioeng 81(3):255–262
Walsh KAJ, Daniel RM, Morgan HW (1983) A soluble NADH dehydrogenase (NADH-ferricyanide oxidoreductase) from Thermus aquaticus strain T351. Biochem J 209(2):427–433
Williamson D, Lund P, Krebs HA (1967) Redox state of free nicotinamide adenine dinucleotide in cytoplasm and mitochondria of rat liver. Biochem J 103(2):514–527
Willquist K (2010) Physiology of Caldicellulosiruptor saccharolyticus: a hydrogen cell factory. Doctoral thesis, Department of Applied Microbiology, Lund University, Lund
Willquist K, van Niel EWJ (2010) Lactate formation in Caldicellulosiruptor saccharolyticus is regulated by the energy carriers pyrophosphate and ATP. Metab Eng 12(3):282–290
Willquist K, Claassen PAM, van Niel EWJ (2009) Evaluation of the influence of CO2 on hydrogen production by Caldicellulosiruptor saccharolyticus. Int J Hydrogen Energy 34(11):4718–4726
Zhang ZQ, Yu J, Stanton RC (2000) A method for determination of pyridine nucleotides using a single extract. Anal Biochem 285(1):163–167
Acknowledgments
The Marie Curie Fellowships for Early Stage Research Training (BIONEL), the EU FP7 NMP project EXCELL, and the EU HYVOLUTION Program, FP6-SES IP (Contract no. 019825) are kindly acknowledged for financial support. We thank Dr. Gabriela Blagoi and Dr. Arto Heiskanen (Department of Micro- and Nanotechnology, Technical University of Denmark) for stimulating and fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
N. Kostesha and K. Willquist contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Kostesha, N., Willquist, K., Emneus, J. et al. Probing the redox metabolism in the strictly anaerobic, extremely thermophilic, hydrogen-producing Caldicellulosiruptor saccharolyticus using amperometry. Extremophiles 15, 77–87 (2011). https://doi.org/10.1007/s00792-010-0341-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-010-0341-4