Abstract
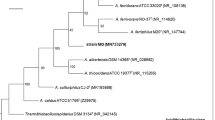
Phenotypic and genotypic analysis was carried out on four iron- and sulfur-oxidizing acidophilic bacteria (the “NO-37 group”) isolated from different parts of the world. 16S rRNA phylogeny showed that they are highly related to each other, but are less related to the type strain of Acidithiobacillus ferrooxidans. The NO-37 group isolates are obligate chemolithoautotrophs, facultative anaerobes, diazotrophic, and psychrotolerant. They are less tolerant of extremely low pH, and in contrast to At. ferrooxidans T, all of the NO-37 group isolates are motile. The GC contents of genomic DNA of the NO-37 group isolates were around 56 mol% and the DNA–DNA hybridization value between genomic DNA of isolate NO-37 and At. ferrooxidans T was 37%. It also appears that the bacteria of the NO-37 group have a different biochemical mechanism for oxidizing ferrous iron than At. ferrooxidans T; the gene coding for the archetypal rusticyanin (RusA) was not detected in any of the NO-37 group isolates, rather a gene coding for a homologous protein (RusB) was amplified from three of the four novel isolates. Isolates of the NO-37 group clearly belong to a species that is different to those already recognized in the genus Acidithiobacillus, for which the name Acidithiobacillus ferrivorans is proposed.




Similar content being viewed by others
References
Blake R, Johnson DB (2000) Phylogenetic and biochemical diversity among acidophilic bacteria that respire on iron. In: Lovley DR (ed) Environmental microbe-metal interactions. American Society of Microbiology Press, Washington, DC, pp 53–78
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Drobner E, Huber H, Stetter KO (1990) Thiobacillus ferrooxidans, a facultative hydrogen oxidizer. Appl Environ Microbiol 56:2922–2923
Drobner E, Huber H, Rachel R, Stetter KO (1992) Thiobacillus plumbophilus sp. nov., a novel galena and hydrogen oxidizer. Arch Microbiol 157:213–217
Ghauri MA, Okibe N, Johnson DB (2007) Attachment of acidophilic bacteria to solid surfaces: the significance of species and strain variations. Hydrometallurgy 85:72–80
Hallberg KB, Lindström EB (1994) Characterization of Thiobacillus caldus sp. nov., a moderately thermophilic acidophile. Microbiology 140:3451–3456
Hallberg KB, Thomson HEC, Boeselt I, Johnson DB (2001) Aerobic and anaerobic sulfur metabolism by acidophilic bacteria. In: Ciminelli VST, Garcia O Jr (eds) Biohydrometallurgy: fundamentals, technology and sustainable development. Elsevier, Amsterdam, pp 423–431
Harrison AP Jr (1982) Genomic and physiological diversity amongst strains of Thiobacillus ferrooxidans, and genomic comparison with Thiobacillus thiooxidans. Arch Microbiol 131:68–76
Holmes DS, Bonnefoy V (2007) Genetic and bioinformatic insights into iron and sulfur oxidation mechanisms of bioleaching organisms. In: Rawlings DE, Johnson DB (eds) Biomining. Springer-Verlag, Berlin, pp 281–307
Johnson DB (1995) Selective solid media for isolating and enumerating acidophilic bacteria. J Microbiol Methods 23:205–218
Johnson DB, Hallberg KB (2007) Techniques for detecting and identifying acidophilic mineral-oxidizing microorganisms. In: Rawlings DE, Johnson DB (eds) Biomining. Springer-Verlag, Berlin, pp 237–261
Johnson DB, Hallberg KB (2008) Carbon, iron and sulfur metabolism in acidophilic micro-organisms. Adv Microb Physiol 54:202–256
Johnson DB, Kelso WI (1983) Detection of heterotrophic contaminants in cultures of Thiobacillus ferrooxidans and their elimination by subculturing in media containing copper sulphate. J Gen Microbiol 123:2969–2972
Johnson DB, Rolfe S, Hallberg KB, Iversen E (2001) Isolation and phylogenetic characterisation of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned Norwegian copper mine. Environ Microbiol 3:630–637
Johnson DB, Bacelar-Nicolau P, Okibe N, Thomas A, Hallberg KB (2009) Characteristics of Ferrimicrobium acidiphilum gen. nov., sp. nov., and Ferrithrix thermotolerans gen. nov., sp. nov.: heterotrophic iron-oxidizing, extremely acidophilic actinobacteria. Int J Syst Evol Microbiol 59:1082–1089
Karavaiko GI, Tourova TP, Kondrat’eva TF, Lysenko AM, Kolganova TV, Ageeva SN, Muntyan LN, Pivovarova TA (2003) Phylogenetic heterogeneity of the species Acidithiobacillus ferrooxidans. Int J Syst Evol Microbiol 53:113–119
Kelly DP, Tuovinen OH (1972) Recommendation that the names Ferrobacillus ferrooxidans Leathen and Braley and Ferrobacillus sulfooxidans Kinsel be recognised as synonyms of Thiobacillus ferrooxidans Temple and Colmer. Int J Syst Bacteriol 22:170–172
Kelly DP, Wood AP (2000) Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. Int J Syst Evol Microbiol 50:511–516
Kelly DP, Wood AP (2005) Genus Acidithiobacillus (Kelly and Wood 2000). In: Brenner DJ, Krieg NR, Staley JT (eds) Bergey’s manual of systematic bacteriology, second edition, volume two the Proteobacteria. Part B. The Gammaproteobacteria. Bergey’s Manual Trust, Michigan, pp 60–62
Kinsel N (1960) New sulfur oxidizing iron bacterium: Ferrobacillus sulfooxidans. J Bacteriol 80:628–632
Kupka D, Rzhepishevska OI, Dopson M, Lindström EB, Karnachuk OV, Tuovinen OH (2007) Bacterial oxidation of ferrous iron at low temperatures. Biotechnol Bioeng 97:1470–1478
Leathen WW, Kinsel NA, Braley SA (1956) Ferrobacillus ferrooxidans: a chemosynthetic autotrophic bacterium. J Bacteriol 72:700–704
Lovley DR, Phillips EJP (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 53:1536–1540
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Mackintosh MEJ (1978) Nitrogen fixation by Thiobacillus ferrooxidans. J Gen Microbiol 105:215–218
Ni YQ, He KY, Bao JT, Wan DS, Li HY (2008) Genomic and phenotypic heterogeneity of Acidithiobacillus spp. strains isolated from diverse habitats in China. FEMS Microbiol Ecol 64:248–259
Nordstrom DK (2000) Advances in the hydrogeochemistry and microbiology of acid mine waters. Int Geol Rev 42:499–515
Norris PR, Murrell JC, Hinson D (1995) The potential for diazotrophy in iron- and sulfur-oxidizing acidophilic bacteria. Arch Microbiol 16:294–300
Okibe N, Gericke M, Hallberg KB, Johnson DB (2003) Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred tank bioleaching operation. Appl Environ Microbiol 69:1936–1943
Pronk JT, Meijer WM, Hazeu W, van Dijken JP, Bos P, Kuenen JG (1991) Growth of Thiobacillus ferrooxidans on formic acid. Appl Environ Microbiol 57:2057–2062
Pronk JT, de Bruyn JC, Bos P, Kuenen JG (1992) Anaerobic growth of Thiobacillus ferrooxidans. Appl Environ Microbiol 58:2227–2230
Rawlings DE, Johnson DB (eds) (2007) Biomining. Springer-Verlag, Berlin
Sasaki K, Ida C, Ando A, Matsumoto N, Saiki H, Ohmura N (2003) Respiratory isozyme, two types of rusticyanin of Acidithiobacillus ferrooxidans. Biosci Biotechnol Biochem 67:1039–1047
Schnaitman CA, Korczynski MS, Lundgren DG (1969) Kinetic studies of iron oxidation by whole cells of Ferrobacillus ferrooxidans. J Bacteriol 99:552–557
Temple KL, Colmer AR (1951) The autotrophic oxidation of iron by a new bacterium: Thiobacillus ferrooxidans. J Bacteriol 62:605–611
Tuovinen OH, Kelly DP (1974) Studies on the growth of Thiobacillus ferrooxidans. V. Factors affecting growth in liquid culture and the development of colonies on solid media containing inorganic sulfur compounds. Arch Microbiol 98:351–364
Ueda T, Suga Y, Yahiro N, Matsuguchi T (2005) Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nifH gene sequences. J Bacteriol 177:1414–1417
Valdés J, Pedroso I, Quatrini R, Holmes DS (2008) Comparative genome analysis of Acidithiobacillus ferrooxidans, A. thiooxidans and A. caldus: insights into their metabolism and ecophysiology. Hydrometallurgy 94:180–184
Wakeman K, Auvinen H, Johnson DB (2008) Microbiological and geochemical dynamics in simulated heap leaching of a polymetallic sulfide ore. Biotechnol Bioeng 101:739–750
Wichlacz PL, Unz RF, Langworthy TA (1986) Acidiphilium angustum sp. nov., Acidiphilium facilis sp. nov., and Acidiphilium rubrum sp. nov.: acidophilic heterotrophic bacteria isolated from acid coal mine drainage. Int J Syst Bacteriol 36:197–201
Acknowledgments
This study was carried out in the frame of BioMinE Project, supported by the European Commission under the Sixth Framework Programme for Research and Development (Contract NMP1-CT-500329-1). We wish to thank our various partners on the project for their contributions to the work reported in this paper. The authors would also like to thank Professor Jean Euzéby for his expert advice on bacterial nomenclature, Dr. Eleanor Jameson for conducting experiments on anaerobic growth of At. ferrivorans, and Mr. Pedro Galleguillos and Dr. Tadayoshi Kanao for measurement of specific iron oxidation rates. D.B.J. is grateful to the Royal Society (UK) for the award of an Industrial Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Matsunaga.
Rights and permissions
About this article
Cite this article
Hallberg, K.B., González-Toril, E. & Johnson, D.B. Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 14, 9–19 (2010). https://doi.org/10.1007/s00792-009-0282-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-009-0282-y