Abstract
Objectives
The purpose of the present study was the evaluation of effectiveness and efficiency of a powder consisting of glycine and tricalcium phosphate in comparison to two established powders based on glycine or sodium bicarbonate in biofilm removal on titanium and zirconium implant surfaces.
Materials and methods
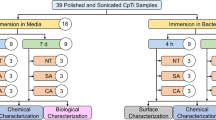
Biofilm was collected for 48 h by five volunteers. A total of 69 titanium and 69 zirconium samples were randomly assigned to test and control groups. Residual plaque areas (RPA) and treatment time were taken as parameters.
Results
Within the titanium groups, mean RPA was determined in the following descending order: sodium bicarbonate > glycine > glycine + tricalcium phosphate. Differences between the groups were significant, p < 0.05. Mean treatment time in the titanium groups was determined in the following descending order without significant differences, p > 0.05: glycine + tricalcium phosphate > sodium bicarbonate > glycine. Regarding the zirconium groups, mean RPA was detected in the following descending order, without significant differences, p > 0.05: glycine > sodium bicarbonate > glycine + tricalcium phosphate. Mean treatment time of the glycine + tricalcium phosphate group was significantly lower than in the control groups, p < 0.05.
Conclusions
It can be concluded that glycine + tricalcium phosphate seemed to be more effective than the control groups for biofilm removal on titanium and zirconium implant surfaces. Especially on zirconium surfaces, decontamination with glycine + tricalcium phosphate seemed to be more efficient than treatment with glycine or sodium bicarbonate.
Clinical relevance
The combination of glycine and tricalcium phosphate could improve the clinical outcomes of air-abrasive device in nonsurgical peri-implantitis therapy.






Similar content being viewed by others
References
Zitzmann NU, Berglundh T (2008) Definition and prevalence of peri-implant diseases. J Clin Periodontol 35(8):286–291
Esposito M, Hirsch J, Lekholm U, Thomsen P (1999) Differential diagnosis and treatment strategies for biologic complications and failing oral implants: a review of the literature. Int J Oral Maxillofac Implants 14(4):473–490
Quirynen M, De Soete M, van Steenberghe D (2002) Infectious risks for oral implants: a review of the literature. Clin Oral Implants Res 13(1):1–19
Leonhardt A, Dahlén G, Renvert S (2003) Five year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J Periodontol 74(10):1415–1422
Mombelli A, Lang NP (1994) Microbial aspects of implant dentistry. Periodontology 2000(4):74–80
Renvert S, Lessem J, Dahlén G, Renvert H, Lindahl C (2008) Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: a randomized clinical trial. J Periodontol 79(5):836–844
Fox SC, Moriarty JD, Kusy RP (1990) The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol 61(8):485–490
Schwarz F, Sculean A, Romanos G, Herten M, Horn N, Scherbaum W, Becker J (2005) Influence of different treatment approaches on the removal of early plaque biofilms and the viability of SAOS2 osteoblasts grown on titanium implants. Clin Oral Investig 9(2):111–117
Mann M, Parmar D, Walmsley AD, Lea SC (2012) Effect of plastic-covered ultrasonic scalers on titanium implant surfaces. Clin Oral Implants Res 23(1):76–82
Augthun M, Tinschert J, Huber A (1998) In vitro studies on the effect of cleaning methods on different implant surfaces. J Periodontol 69(8):857–864
Kreisler M, Kohnen W, Christoffers AB, Gotz H, Jansen B, Duschner H, d’Hoedt B (2005) In vitro evaluation of the biocompatibility of contaminated implant surfaces treated with an Er: YAG laser and an air powder system. Clin Oral Implants Res 16(1):36–43
Parham Jr PL, Cobb CM, French AA, Love JW, Drisko CL, Killoy WJ (1989) Effects of an air-powder abrasive system on plasma-sprayed titanium implant surfaces: an in vitro evaluation. J Oral Implantol 15(2):78–86
Matarasso S, Quaremba G, Coraggio F, Vaia E, Cafiero C, Lang NP (1996) Maintenance of implants: an in vitro study of titanium implant surface modifications subsequent to the application of different prophylaxis procedures. Clin Oral Implants Res 7(1):64–72
Meschenmoser A, d’Hoedt B, Meyle J, Elssner G, Korn D, Hämmerle H, Schulte W (1996) Effects of various hygiene procedures on the surface characteristics of titanium abutments. J Periodontol 67(3):229–235
Schwarz F, Ferrari D, Popovski K, Hartig B, Becker J (2009) Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J Biomed Mater Res B Appl Biomater 88(1):83–91
Tastepe CS, Liu Y, Visscher CM, Wismeijer D (2013) Cleaning and modification of intraorally contaminated titanium discs with calcium phosphate powder abrasive treatment. Clin Oral Implants Res 24(11):1238–1246
Hannink G, Arts JJ (2011) Bioresorbability, porosity and mechanical strength of bone substitutes: what is optimal for bone regeneration? Injury 42(2):22–25
Auschill TM, Arweiler NB, Netuschil L, Brecx M, Reich E, Sculean A (2001) Spatial distribution of vital and dead microorganisms in dental biofilms. Arch Oral Biol 46(5):471–476
Schwarz F, Sculean A, Romanos G, Herten M, Horn N, Scherbaum W, Becker J (2005) Influence of different treatment approaches on the removal of early plaque biofilms and the viability of SAOS2 osteoblasts grown on titanium implants. Clin Oral Investig 9(2):111–117
John G, Becker J, Schwarz F (2013) Modified implant surface with slower and less initial biofilm formation. Clin Implant Dent Relat Res 17(3):461–468
Schwarz F, Sculean A, Wieland M, Horn N, Nuesry E, Bube C, Becker J (2007) Effects of hydrophilicity and microtopography of titanium implant surfaces on initial supragingival plaque biofilm formation. A pilot study. Mund Kiefer Gesichtschir 11(6):333–338
John G, Schwarz F, Becker J (2014) Taurolidine as an effective and biocompatible additive for plaque-removing techniques on implant surfaces. Clin Oral Investig 19(5):1069–1077
Siegrist BE, Brecx MC, Gusberti FA, Joss A, Lang NP (1991) In vivo early human dental plaque formation on different supporting substances. A scanning electron microscopic and bacteriological study. Clin Oral Implants Res 2(1):38–46
Rimondini L, Fare S, Brambilla E, Felloni A, Consonni C, Brossa F, Carrassi A (1997) The effect of surface roughness on early in vivo plaque colonization on titanium. J Periodontol 68(6):556–562
Sennerby L, Lekholm U (1993) The soft tissue response to titanium abutments retrieved from humans and reimplanted in rats. A light microscopic study. Clin Oral Implants Res 4(1):23–27
Mouhyi J, Sennerby L, Wennerberg A, Louette P, Dourov N, van Reck J (2000) Re-establishment of the atomic composition and the oxide structure of contaminated titanium surfaces by means of carbon dioxide laser and hydrogen peroxide: an in vitro study. Clin Implant Dent Relat Res 2(4):190–202
Schwarz F, Papanicolau P, Rothamel D, Beck B, Herten M, Becker J (2006) Influence of plaque biofilm removal on reestablishment of the biocompatibility of contaminated titanium surfaces. J Biomed Mater Res A 77(3):437–444
John G, Becker J, Schwarz F (2014) Rotating titanium brush for plaque removal from rough titanium surfaces-an in vitro study. Clin Oral Implants Res 25(7):838–842
Shibli JA, Silverio KG, Martins MC, Marcantonio Jr E, Rossa Jr C (2003) Effect of an air-powder system on titanium surface on fibroblast adhesion and morphology. Implant Dent 12(1):81–86
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
John, G., Becker, J. & Schwarz, F. Effectivity of air-abrasive powder based on glycine and tricalcium phosphate in removal of initial biofilm on titanium and zirconium oxide surfaces in an ex vivo model. Clin Oral Invest 20, 711–719 (2016). https://doi.org/10.1007/s00784-015-1571-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1571-8