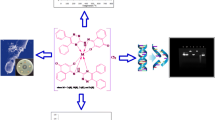
Abstract
In this work synthesis, characterization and crystal structures of 1, Zn(II) complex ([ZnL1(NCS)2]), with (E)-1-(2-oxo-2-(2-(quinolin-2-ylmethylene)hydrazinyl)ethyl)pyridin-1-ium chloride (HL1Cl) and 2, Bi(III) complex ([BiHL2Cl4] × 1/2CH3OH), with (E)-N,N,N-trimethyl-2-oxo-2-(2-(1-(thiazol-2-yl)ethylidene)hydrazinyl)ethan-1-aminium chloride (HL2Cl), have been reported. Zn(II) complex possesses a distorted trigonal bipyramidal geometry while surroundings around Bi(III) ion are extended pentagonal bipyramidal. Antimicrobial activity, brine shrimp assay and DPPH radical scavenging activity of both complexes, including previously synthesized complexes with HL2Cl ligand (Zn(II) and Ni(II)) and complexes with (E)-N,N,N-trimethyl-2-oxo-2-(2-(1-(pyridin-2-yl)ethylidene)hydrazinyl)ethan-1-aminium chloride (HL3Cl) (Zn(II), Cu(II), Cd(II), Co(II), Fe(III), Ni(II)), were evaluated. For the most active complexes, cytotoxic activity against five malignant cancer cell lines (HeLa, A375, MCF7, PC-3 and A549) and normal cell line HaCaT, as well as generation of reactive oxygen species (ROS), was tested.
Graphic abstract









Similar content being viewed by others
References
Qin W, Long S, Panunzio M, Biondi S (2013) Schiff bases: a short survey on an evergreen chemistry tool. Molecules 18:12264–12289. https://doi.org/10.3390/molecules181012264
Shakdofa MME, Shtaiwi MH, Morsy N, Abdel-rassel TMA (2014) Metal complexes of hydrazones and their biological, analytical and catalytic applications: a review. Main Group Chem 13:187–218. https://doi.org/10.3233/MGC-140133
Belkheiri N, Bouguerne B, Bedos-Belval F et al (2010) Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur J Med Chem 45:3019–3026. https://doi.org/10.1016/j.ejmech.2010.03.031
Kaushik D, Khan SA, Chawla G, Kumar S (2010) N’-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene] 2/4-substituted hydrazides: synthesis and anticonvulsant activity. Eur J Med Chem 45:3943–3949. https://doi.org/10.1016/j.ejmech.2010.05.049
Júnior WB, Alexandre-Moreira MS, Alves MA et al (2011) Analgesic and anti-inflammatory activities of salicylaldehyde 2-chlorobenzoyl hydrazone (H2LASSBio-466), salicylaldehyde 4-chlorobenzoyl hydrazone (H2LASSBio-1064) and their Zinc(II) complexes. Molecules 16:6902–6915. https://doi.org/10.3390/molecules16086902
Catto M, Aliano R, Carotti A et al (2010) Design, synthesis and biological evaluation of indane-2-arylhydrazinylmethylene-1,3-diones and indol-2-aryldiazenylmethylene-3-ones as β-amyloid aggregation inhibitors. Eur J Med Chem 45:1359–1366. https://doi.org/10.1016/j.ejmech.2009.12.029
Altintop MD, Sever B, Eklioğlu ÖA et al (2020) A series of furan-based hydrazones: design, synthesis, and evaluation of antimicrobial activity, cytotoxicity and genotoxicity. Lett Drug Des Discov 17:312–322. https://doi.org/10.2174/1570180816666190325163948
Kocyigit-Kaymakcioglu B, Yazici SS, Tok F et al (2019) Synthesis and anticancer activity of new hydrazide-hydrazones and their Pd(II) complexes. Lett Drug Des Discov 16:522–532. https://doi.org/10.2174/1570180815666180816124102
Rollas S, Küçükgüzel S (2007) Biological activities of hydrazone derivatives. Molecules 12:1910–1939. https://doi.org/10.3390/12081910
Alam M, Verma G, Shaquiquzzaman M et al (2014) A review exploring biological activities of hydrazones. J Pharm Bioallied Sci 6:69–80. https://doi.org/10.4103/0975-7406.129170
Adsule S, Barve V, Chen D et al (2006) Novel Schiff base copper complexes of quinoline-2 carboxaldehyde as proteasome inhibitors in human prostate cancer cells. J Med Chem 49:7242–7246. https://doi.org/10.1021/jm060712l
Cukierman DS, Accardo E, Gomes RG et al (2018) Aroylhydrazones constitute a promising class of ‘metal-protein attenuating compounds’ for the treatment of Alzheimer’s disease: a proof-of-concept based on the study of the interactions between zinc(II) and pyridine-2-carboxaldehyde isonicotinoyl hydrazone. J Biol Inorg Chem 23:1227–1241. https://doi.org/10.1007/s00775-018-1606-0
Çınarlı M, Yüksektepe Ataol Ç, Çınarlı E, İdil Ö (2020) Synthesis, characterization, biological, X-ray diffraction analysis and computational chemistry studies of new 2-acetylpyridine derivative hydrazone and its Zn(II) complex. J Mol Struct 1213:128–152. https://doi.org/10.1016/j.molstruc.2020.128152
Abouzayed FI, Emam SM, Abouel-Enein SA (2020) Synthesis, characterization and biological activity of nano-sized Co(II), Ni(II), Cu(II), Pd(II) and Ru(III) complexes of tetradentate hydrazone ligand. J Mol Struct 1216:128–314. https://doi.org/10.1016/j.molstruc.2020.128314
Vojinović-Ješić LS, Bogdanović GA, Leovac VM et al (2008) Transition metal complexes with Girard reagent-based ligands. Part IV. Synthesis and characterization of pyridoxilidene Girard-T hydrazone complexes. Crystal structure of the copper(II) complex. Struct Chem 19:807–881. https://doi.org/10.1007/s11224-008-9368-x
Vojinovic-Jesic L, Novakovic S, Leovac V, Cesljevic V (2012) Transition metal complexes with Girard reagents and their hydrazones. J Serb Chem Soc 77:1129–1155. https://doi.org/10.2298/JSC120704083V
Milenković MR, Čobeljić B, Anđelković K, Turel I (2018) Molecular structures and spin-states of pseudohalide metal complexes with hydrazones of Girard’s T Reagent. Eur J Inorg Chem 2018:838–846. https://doi.org/10.1002/ejic.201701387
Sedaghat T, Tarassoli A, Ansari-Asl Z, Motamedi H (2013) Water soluble organotin(IV) complexes with Girard-T reagent-based hydrazones: synthesis, spectral characterization, and antibacterial activity. J Coord Chem 66:2549–2557. https://doi.org/10.1080/00958972.2013.809424
Yang N, Sun H (2007) Biocoordination chemistry of bismuth: recent advances. Coord Chem Rev 251:2354–2366. https://doi.org/10.1016/j.ccr.2007.03.003
Yang Y, Ouyang R, Xu L et al (2015) Review: Bismuth complexes: synthesis and applications in biomedicine. J Coord Chem 68:379–397. https://doi.org/10.1080/00958972.2014.999672
Kowalik M, Masternak J, Barszcz B (2019) Recent research trends on bismuth compounds in cancer chemoand radiotherapy. Curr Med Chem 26:729–759. https://doi.org/10.2174/0929867324666171003113540
Tiekink ERT (2002) Antimony and bismuth compounds in oncology. Crit Rev Oncol Hematol 42:217–224. https://doi.org/10.1016/S1040-8428(01)00217-7
Ferreira IP, Piló EDL, Recio-Despaigne AA et al (2016) Bismuth(III) complexes with 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones: antimicrobial and cytotoxic activities and effects on the clonogenic survival of human solid tumor cells. Bioorg Med Chem 24:2988–2998. https://doi.org/10.1016/j.bmc.2016.05.007
Ferraz KSO, Silva NF, da Silva JG et al (2012) Investigation on the pharmacological profile of 2,6-diacetylpyridine bis(benzoylhydrazone) derivatives and their antimony(III) and bismuth(III) complexes. Eur J Med Chem 53:98–106. https://doi.org/10.1016/j.ejmech.2012.03.040
Auld DS (2001) Zinc coordination sphere in biochemical zinc sites. Biometals 14:271–313. https://doi.org/10.1023/A:1012976615056
Tian X, Hussain S, de Pace C et al (2019) ZnII complexes for bioimaging and correlated applications. Chem Asian J 14:509–526. https://doi.org/10.1002/asia.201900091
Dasgupta S, Karim S, Banerjee S et al (2020) Designing of novel Zinc(II) Schiff base complexes having acyl hydrazone linkage: study of phosphatase and anti-cancer activity. Dalton Trans 49:1232–1240. https://doi.org/10.1039/C9DT04636D
Crichton RR (2012) Biological inorganic chemistry, 2nd edn. Elsevier, Amsterdam
Qi G, Yang Z, Wang B (2007) Synthesis, characterization and DNA-binding properties of zinc(II) and nickel(II) Schiff base complexes. Transit Met Chem 32:233–239. https://doi.org/10.1007/s11243-006-0160-8
Deo K, Pages B, Ang D et al (2016) Transition metal intercalators as anticancer agents—recent advances. Int J Mol Sci 17:1818–1835. https://doi.org/10.3390/ijms17111818
Brađan G, Pevec A, Turel I et al (2016) Synthesis, characterization, DFT calculations and antimicrobial activity of pentagonal-bipyramidal Zn(II) and Cd(II) complexes with 2,6-diacetylpyridine-bis(trimethylammoniumacetohydrazone). J Coord Chem 69:2754–2765. https://doi.org/10.1080/00958972.2016.1212339
Romanović MČ, Čobeljić B, Pevec A et al (2017) Synthesis, characterization, DFT calculations and antimicrobial activity of Cd(II) complexes with the condensation product of 2-quinolinecarboxaldehyde and Girard’s T reagent. J Coord Chem 70:3702–3714. https://doi.org/10.1080/00958972.2017.1405262
Anđelković K, Milenković MR, Pevec A et al (2017) Synthesis, characterization and crystal structures of two pentagonal-bipyramidal Fe(III) complexes with dihydrazone of 2,6-diacetylpyridine and Girard’s T reagent. Anticancer properties of various metal complexes of the same ligand. J Inorg Biochem 174:137–149. https://doi.org/10.1016/j.jinorgbio.2017.06.011
Romanović MČ, Čobeljić B, Pevec A et al (2017) Synthesis, crystal structures and antimicrobial activity of azido and isocyanato Zn(II) complexes with the condensation product of 2-quinolinecarboxaldehyde and Girard’s T reagent. J Coord Chem 70:2425–2435. https://doi.org/10.1080/00958972.2017.1343945
Čobeljić B, Pevec A, Stepanović S et al (2018) Structural diversity of isothiocyanato Cd(II) and Zn(II) Girard’s T hydrazone complexes in solution and solid state: effect of H-bonding on coordination number and supramolecular assembly of Cd(II) complex in solid state. Struct Chem 29:1797–1806. https://doi.org/10.1007/s11224-018-1155-8
Adejumo TT, Tzouras NV, Zorba LP et al (2020) Synthesis, characterization, catalytic activity, and DFT calculations of Zn(II) hydrazone complexes. Molecules 25:4043–4060. https://doi.org/10.3390/molecules25184043
Cobeljic B, Pevec A, Jaglicic Z et al (2018) Synthesis, characterization and antimicrobial activity of isothiocyanato Fe(III) Girard’s t hydrazone complex. J Serb Chem Soc 83:1327–1337. https://doi.org/10.2298/JSC180828079C
Čobeljić B, Turel I, Pevec A et al (2018) Synthesis, structures and magnetic properties of octahedral Co(III) complexes of heteroaromatic hydrazones with tetraisothiocyanato Co(II) anions. Polyhedron 155:425–432. https://doi.org/10.1016/j.poly.2018.08.070
Keškić T, Čobeljić B, Gruden M et al (2019) what is the nature of interactions of BF4 −, NO3 −, and ClO4 − to Cu(II) complexes with Girard’s T hydrazine? When can binuclear complexes be formed? Cryst Growth Des 19:4810–4821. https://doi.org/10.1021/acs.cgd.9b00760
Milenković MR, Papastavrou AT, Radanović D et al (2019) Highly-efficient N-arylation of imidazole catalyzed by Cu(II) complexes with quaternary ammonium-functionalized 2-acetylpyridine acylhydrazone. Polyhedron 165:22–30. https://doi.org/10.1016/j.poly.2019.03.001
Keskic T, Radanovic D, Pevec A et al (2020) Synthesis, X-ray structure and DFT calculation of magnetic properties of binuclear Ni(II) complex with tridentate hydrazone-based ligand. J Serb Chem Soc 85:1279–1290. https://doi.org/10.2298/JSC200625038K
Keškić T, Jagličić Z, Pevec A et al (2020) Synthesis, X-ray structures and magnetic properties of Ni(II) complexes of heteroaromatic hydrazone. Polyhedron 191:114802–114814. https://doi.org/10.1016/j.poly.2020.114802
Afkhami FA, Khandar AA, Mahmoudi G et al (2016) Synthesis, X-ray characterization, DFT calculations and Hirshfeld surface analysis of Zn(II) and Cd(II) complexes based on isonicotinoylhydrazone ligand. CrystEngComm 18:4587–4596. https://doi.org/10.1039/C6CE00877A
Afkhami FA, Mahmoudi G, Khandar AA et al (2019) Design and construction of Zn(II) coordination polymers made by pincer type pyridine-hydrazine based ligands. J Mol Struct 1197:555–563. https://doi.org/10.1016/j.molstruc.2019.07.090
Scilabra P, Terraneo G, Resnati G (2019) The chalcogen bond in crystalline solids: a world parallel to halogen bond. Acc Chem Res 52:1313–1324. https://doi.org/10.1021/acs.accounts.9b00037
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The Cambridge structural database. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater 72:171–179. https://doi.org/10.1107/S2052520616003954
Starbuck J, Norman NC, Guy Orpen A (1999) Secondary bonding as a potential design element for crystal engineering. New J Chem 23:969–972. https://doi.org/10.1039/a906352h
Kyriakidis CE, Christidis PC, Rentzeperis PJ et al (1990) Crystal structure and spectra of Trichloro-p-chlorobenzoyl-2-furaldehydohydrazono bismuth(III). Z Kristallogr 193:101–110. https://doi.org/10.1524/zkri.1990.193.1-2.101
Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM (2019) Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med 9:84–105. https://doi.org/10.3390/jcm9010084
Szekely J, Gates KS (2006) Noncovalent DNA binding and the mechanism of oxidative DNA damage by fecapentaene-12. Chem Res Toxicol 19:117–121. https://doi.org/10.1021/tx050197e
Li X-L, Hu Y-J, Wang H et al (2012) Molecular spectroscopy evidence of berberine binding to DNA: comparative binding and thermodynamic profile of intercalation. Biomacromol 13:873–880. https://doi.org/10.1021/bm2017959
Vijayalakshmi R, Kanthimathi M, Subramanian V, Nair BU (2000) DNA cleavage by a chromium(III) complex. Biochem Biophys Res Commun 271:731–734. https://doi.org/10.1006/bbrc.2000.2707
Kakkar R, Garg R, Suruchi A (2002) Towards understanding the molecular recognition process in Hoechst–DNA complexes. J Mol Struct (Thoechem) 584:37–44. https://www.scopus.com/authid/detail.uri?authorId=7409949663
Lakowicz JR, Weber G (1973) Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 12:4161–4170. https://doi.org/10.1021/bi00745a020
Sheldrick GM (2015) SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr Sect A Found Adv 71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71:3–8. https://doi.org/10.1107/S2053229614024218
Dolomanov OV, Bourhis LJ, Gildea RJ et al (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Sartoratto A, Machado ALM, Delarmelina C et al (2004) Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol 35:275–280. https://doi.org/10.1590/S1517-83822004000300001
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Ohno M, Abe T (1991) Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6). J Immunol Methods 145:199–203. https://doi.org/10.1016/0022-1759(91)90327-C
Matić IZ, Aljančić I, Žižak Ž et al (2013) In vitro antitumor actions of extracts from endemic plant Helichrysum zivojinii. BMC Complement Altern Med 13:36–48. https://doi.org/10.1186/1472-6882-13-36
Ormerod MG (2000) Flow cytometry. A practical approach, 3rd edn. Oxford University Press
Mihailović N, Marković V, Matić IZ et al (2017) Synthesis and antioxidant activity of 1,3,4-oxadiazoles and their diacylhydrazine precursors derived from phenolic acids. RSC Adv 7:8550–8560. https://doi.org/10.1039/C6RA28787E
Aranda E, Owen GI (2009) A semi-quantitative assay to screen for angiogenic compounds and compounds with angiogenic potential using the EA.hy926 endothelial cell line. Biol Res 42:377–389. https://doi.org/10.4067/S0716-97602009000300012
Matić IZ, Aljančić I, Vajs V et al (2013) Cancer-suppressive potential of extracts of endemic plant Helichrysum zivojinii: effects on cell migration, invasion and angiogenesis. Nat Prod Commun 8:1291–1296
Reichmann ME, Rice SA, Thomas CA, Doty P (1954) a further examination of the molecular weight and size of desoxypentose nucleic acid. J Am Chem Soc 76:3047–3053. https://doi.org/10.1021/ja01640a067
Dower WJ, Miller JF, Ragsdale CW (1988) High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res 16:6127–6145. https://doi.org/10.1093/nar/16.13.6127
Acknowledgements
The authors are grateful to the Ministry of Education, Science and Technological Development of the Republic of Serbia for the financial support (Grant numbers: 451-03-9/2021-14/20004, 451-03-68/2021-14/200026 and 451-03-9/2021-14/200168). The Laboratorio di Strutturistica “M. Nardelli” of the University of Parma and Chiesi Farmaceutici SpA are thanked for the X-ray diffraction data collection. This work has benefited from the equipment and framework of the COMP-HUB Initiative, funded by the “Departments of Excellence” program of the Italian Ministry for Education, University and Research (MIUR, 2018-2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stevanović, N., Mazzeo, P.P., Bacchi, A. et al. Synthesis, characterization, antimicrobial and cytotoxic activity and DNA-binding properties of d-metal complexes with hydrazones of Girard’s T and P reagents. J Biol Inorg Chem 26, 863–880 (2021). https://doi.org/10.1007/s00775-021-01893-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-021-01893-5