Abstract
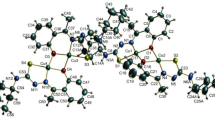
The monoligand complexes of the formula M(HPLGT)(NCS)2 (M = Cu(II), Zn(II)) in which the ligand tridentate ONO pyridoxilidene Girard-T hydrazone, [H3PLGT]Cl2 · 2H2O, was coordinated in neutral doubly deprotonated form were synthesized. Also, the first complexes with the ligand coordinated in triply deprotonated monoanionic form of the formula [Cu(PLGT)N3] and [Co(PLGT)(NO2)2NH3] · 3H2O are reported. The single crystal X-ray analysis of [Cu(HPLGT)(NCS)2] showed that Cu(II) is placed in a square-pyramidal surrounding consisting of one tridentate Schiff base and one NCS group in the basal plane and the other NCS group in the apical position. Intermolecular hydrogen bonds leading to centrosymmetrical dimerization of these complexes were discussed. In the reaction of Girard-T and Hacac in the presence of CuCl2, a mixture of single crystal complexes of the composition [Cu(3,5-Me2pz)2Cl2]2 and [Cu(acac)2] · 2[Cu(3,5-Me2pz)2Cl2] was obtained and X-ray analysis of the latter one was reported.
Index abstract
Crystal structure of the Cu(II) complex with pyridoxilidene Girard-T hydrazone was analyzed. Additional two Cu(II) complexes obtained by the reaction of Girard-T reagent and Hacac in the presence of CuCl2 were also studied by single crystal X-ray analysis. 



Similar content being viewed by others
References
Girard A, Sandulesco G (1936) Helv Chim Acta 19:109. doi:10.1002/hlca.193601901148
Viscontini M, Meier J (1950) Helv Chim Acta 33:1773. doi:10.1002/hlca.19500330646
Wheeler OH (1962) Chem Rev 62:205. doi:10.1021/cr60217a002
Masui M, Ohmori H (1964) Chem Pharm Bull (Tokyo) 12:877
Masui M, Ohmori H (1967) J Chem Soc B:762. doi:10.1039/j29670000762
Wheeler OH (1968) J Chem Educ 45:435
Mostafa MM, Hassan SM, Ibrahim GM (1980) J Inorg Nucl Chem 42:285. doi:10.1016/0022-1902(80)80259-4
Emam MEM, Hafez MA, Moussa MNH (1991) J Therm Anal 37:1005. doi:10.1007/BF01932798
Wang X, Zhang XM, Liu HX (1994) J Coord Chem 33:223. doi:10.1080/00958979408024280
Wang X, Zhang XM, Liu HX (1994) Inorg Chim Acta 223:193. doi:10.1016/0020-1693(94)04007-9
Vojinović LS, Leovac VM, Novaković SB, Bogdanović GA, Csanádi JJ, Češljević VI (2004) Inorg Chem Commun 7:1264. doi:10.1016/j.inoche.2004.09.016
Leovac VM, Mészáros-Szécsényi K, Vojinović-Ješić LS, Češljević VI, Markov S, Wadsten T (2006) J Therm Anal Calorim 86:379. doi:10.1007/s10973-005-7402-4
Leovac VM, Bogdanović GA, Češljević VI, Jovanović LS, Novaković SB, Vojinović-Ješić LS (2007) Struct Chem 18:113. doi:10.1007/s11224-006-9136-8
Mostafa MM, Khattab MA, Ibrahim KM (1983) Transit Metal Chem 8:212. doi:10.1007/BF00620692
El-Bahnasawy RM (1995) J Therm Anal 45:1547. doi:10.1007/BF02547448
Abou Sekkina MM, Salem MR (1997) J Thermal Anal 48:841 and references therein
Enraf-Nonius CAD-4 Software (1989) Version 5.0, Enraf-Nonius. Delft, The Netherlands
CAD-4 Express Software (1994) Enraf-Nonius. Delft, The Netherlands
(a) Sheldrick GM (1997) SHELXS97. Program for the solution of crystal structures. University of Göttingen, Germany; (b) Sheldrick GM (1997) SHELXL97. Program for the refinement of crystal structures. University of Göttingen, Germany
(a) Spek AL (1990) Acta Crystallogr A 46:C34; (b) Spek AL (1998) PLATON: Multipurpose crystallographic tool. Utrecht University, Utrecht, The Netherlands
Farrugia LJ (1999) J Appl Cryst 32:837. doi:10.1107/S0021889899006020
Nardelli M (1995) J Appl Cryst 28:659. doi:10.1107/S0021889895007138
Farrugia LJ (1997) J Appl Cryst 30:565. doi:10.1107/S0021889897003117
Neyding AB (1970) Magnetokhimiya kompleksnykh soedinenii perekhodnykh metallov. Itogi Nauki, Moskva
Nakamoto K (1997) Infrared and raman spectra of inorganic and coordination compounds. Wiley–Interscience, New York
Geary WJ (1971) Coord Chem Rev 7:81. doi:10.1016/S0010-8545(00)80009-0
Bindu P, Pratapachandra Kurup MR (1997) Transit Metal Chem 22:578. doi:10.1023/A:1018512708055
El-Sawaf AK, West DX, El-Saied FA, El-Bahnasawy RM (1997) Transit Metal Chem 22:360. doi:10.1023/A:1018598302178
Sevilla JM, Cambron G, Pineda T, Blázquez M (1995) J Electroanal Chem 381:179. doi:10.1016/0022-0728(94)03695-Y
Lever ABP (1987) Inorganic electronic spectroscopy (Russian translation). Mir, Moskva, p 2
Leovac VM, Jovanović LS, Jevtović VS, Pelosi G, Bisceglie F (2007) Polyhedron 26:2971. doi:10.1016/j.poly.2007.01.041
Nishio M, Hirota M, Umezawa Y (1998) The CH/π Interaction evidence nature and consequences. John Wiley & Sons Inc., New York
Desiraju GR, Steiner T (1999) The weak hydrogen bonds in structural chemistry and biology, Oxford University Press
Bogdanović GA, Spasojević-de Biré A, Zarić SD (2002) Eur J Inorg Chem 1599. doi :10.1002/1099-0682(200207)2002:7<1599::AID-EJIC1599>3.0.CO;2-I
Starikova ZA, Shugam EA (1969) Zh Strukt Khim Russ 10:290
Chandrasekhar V, Kingsley S, Vij A, Lam KC KC, Rheingold AL (2000) Inorg Chem 39:3238. doi:10.1021/ic991255k
Vogel AI, Tatchell AR, Furnis BS, Hannaford AJ, Smith PWG (1989) Vogel’s textbook of practical organic chemistry, 5th edn. Longman, London
Mészáros-Szécsényi K, Leovac VM, Jaćimović ŽK, Češljević VI, Kovács A, Pokol G (2001) J Therm Anal Calorim 66:573. doi:10.1023/A:1013133405261
Mészáros-Szécsényi K, Leovac VM, Češljević VI, Kovács A, Pokol G, Gy Argay, Kálmán A, Bogdanović GA, Jaćimović ŽK, Spasojević-de Biré A (2003) Inorg Chim Acta 353:253. doi:10.1016/S0020-1693(03)00231-7
Acknowledgments
This work was supported by the Ministry of Science and Environmental Protection of the Republic of Serbia (Grant No. 142028) and the Provincial Secretariat for Science and Technological Development of Vojvodina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vojinović-Ješić, L.S., Bogdanović, G.A., Leovac, V.M. et al. Transition metal complexes with Girard reagent-based ligands. Part IV. Synthesis and characterization of pyridoxilidene Girard-T hydrazone complexes. Crystal structure of the copper(II) complex. Struct Chem 19, 807–815 (2008). https://doi.org/10.1007/s11224-008-9368-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-008-9368-x