Abstract
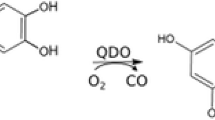
Manganese-dependent homoprotocatechuate 2,3-dioxygenase (MndD) is an enzyme taking part in the catabolism of aromatic compounds in the environment. It uses molecular oxygen to perform an extradiol cleavage of the ring of the ortho-dihydroxylated aromatic compound homoprotocatechuate. A theoretical investigation of the reaction path for MndD was performed using hybrid density functional theory with the B3LYP functional, and a catalytic mechanism has been suggested. Models of different size were built from the crystal structure of the enzyme and were used in the search for intermediates and transition states. It was found that the substrate first binds at the active site as a monoanion. Next the dioxygen is bound, forming a hydroperoxo intermediate. The O–O bond, activated in this way undergoes homolytic cleavage leading to an oxyl and then to an extra epoxide radical with subsequent opening of the aromatic ring. The lactone ring is then hydrolyzed by the Mn-bound OH group, and the final product is obtained in the last reaction steps. Alternative reaction paths were considered, and their calculated barriers were found to be higher than for the suggested mechanism. The selectivity between the extra- and intra-cleavage pathways was found to be determined by the barriers for the decay of the radical state.
























Similar content being viewed by others
References
Ornston LN, Stanier RY (1966) J Biol Chem 241:3776–3786
Dagley S (1977) Surv Prog Chem 8:121–170
Broderick JB (1999) Essays Biochem 34:173–189
Lipscomb JD, Orville AM (1992) Met Ions Biol Syst 28:243–298
Que L Jr, Ho RYN (1996) Chem Rev 96:2607–2624
Boldt YR, Sadowsky MJ, Ellis LBM, Que L Jr, Wackett LP (1995) J Bacteriol 177:1225–1232
Vetting MW, Wackett LP, Que L Jr, Lipscomb JD, Ohlendorf DH (2004) J Bacteriol 186:1945–1958
Bassan A, Borowski T, Siegbahn PEM (2004) Dalton Trans 3153–3162
Que L Jr (2000) Nat Struct Biol 7:182–184
Whiting AK, Boldt YR, Hendrich LP, Wackett LP, Que L Jr (1996) Biochemistry 35:160–170
Sato N, Uragami Y, Nishizaki T, Takahashi Y, Sazaki G, Sugimoto K, Nokana T, Masai E, Fukuda M, Senda T (2002) J Mol Biol 321:621–636
Deeth RJ, Bugg TDH (2003) J Biol Inorg Chem 8:409
Bugg TDH, Lin G (2001) Chem Commun 11:941–952
Miller MA, Lipscomb JD (1996) J Biol Chem 271:5524–5535
Shu L, Chiou YM, Orville AM, Miller MA, Lipscomb JD, Que L Jr (1995) Biochemistry 34:6649–6659
Groce SL, Lipscomb JD (2005) Biochemistry 44:7175–7188
Arciero DM, Orville AM, Lipscomb JD (1985) J Biol Chem 260:14035–14044
Emerson J, Wagner ML, Reynolds MF, Que L Jr, Sadowsky MJ, Wacket LP (2005) J Biol Inorg Chem 10:751–760
Schrödinger Inc (2000) JAGUAR 4.0. Schrödinger, Portland
Frisch MJ et al (2003) Gaussian 03, revision B.03. Gaussian, Pittsburgh
Himo F (2005) Biochim Biophys Acta Bioenerg 1707:24–33
Siegbahn PEM, Haeffner F (2004) J Am Chem Soc 126:8919–8932
Borowski T, Georgiev V, Siegbahn PEM (2005) J Am Chem Soc 127:17303–17314
Bugg TDH (2003) Tetrahedron 59:7075–7101
Shaik S, Kumar D, de Viser S, Altun A, Thiel W (2005) Chem Rev 105:2279–2328
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Georgiev, V., Borowski, T. & Siegbahn, P.E.M. Theoretical study of the catalytic reaction mechanism of MndD. J Biol Inorg Chem 11, 571–585 (2006). https://doi.org/10.1007/s00775-006-0106-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-006-0106-9