Abstract
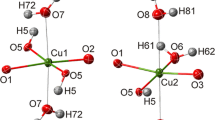
Using single-crystal X-ray diffraction at 293, 200 and 100 K, and neutron diffraction at 50 K, we have refined the positions of all atoms, including hydrogen atoms (previously undetermined), in the structure of coquimbite (\( P {\bar 3}1c \), a = 10.924(2)/10.882(2) Å, c = 17.086(3) / 17.154(3) Å, V = 1765.8(3)/1759.2(5) Å3, at 293 / 50 K, respectively). The use of neutron diffraction allowed us to determine precise and accurate hydrogen positions. The O–H distances in coquimbite at 50 K vary between 0.98 and 1.01 Å. In addition to H2O molecules coordinated to the Al3+ and Fe3+ ions, there are rings of six “free” H2O molecules in the coquimbite structure. These rings can be visualized as flattened octahedra with the distance between oxygen and the geometric center of the polyhedron of 2.46 Å. The hydrogen-bonding scheme undergoes no changes with decreasing temperature and the unit cell shrinks linearly from 293 to 100 K. A review of the available data on coquimbite and its “dimorph” paracoquimbite indicates that paracoquimbite may form in phases closer to the nominal composition of Fe2(SO4)3·9H2O. Coquimbite, on the other hand, has a composition approximating Fe1.5Al0.5(SO4)3·9H2O. Hence, even a “simple” sulfate Fe2-x Al x (SO4)3·9H2O may be structurally rather complex.

Similar content being viewed by others
References
Ackermann S, Armbruster T, Lazic B, Doyle S, Grevel K-D, Majzlan J (2009) Thermodynamic and crystallographic properties of kornelite (Fe2(SO4)3·∼7.75H2O) and paracoquimbite (Fe2(SO4)3·9H2O). Am Mineral 94:1620–1628
Anderson JL, Peterson RC, Swainson IP (2007) The atomic structure and hydrogen bonding of deuterated melanterite, FeSO4·7D2O. Can Mineral 45:457–469
Bretsznajder S, Rojkowski Z (1969) X-ray analysis of certain aluminum sulfate hydrates. B Acad Pol Sci-Chim 17:133–137
Brown ID (2002) The chemical bond in inorganic chemistry. IUCr Monogr Crystallogr 12
Demartin F, Castellano C, Gramaccioli C, Campostrini I (2010) Aluminum/iron substitution, hydrogen bonding and a novel structural type in coquimbite-like minerals. Can Mineral 48:323–333
Fang JH, Robinson PD (1970) Crystal structures and mineral chemistry of hydrated ferric sulfates. I. The crystal structure of coquimbite. Am Mineral 55:1534–1540
Fischer WE (1997) SINQ—the spallation neutron source, a new research facility at PSI. Physica B 234–236:1202–1208
Franks F (eds) (1973) Water: a comprehensive treatise, vol 2. Plenum
Gancy AB (1982) Preparation and characterization of the nonahydrate and pentahydrate of aluminum sulfate. Thermochim Acta 54:105–114
Giacovazzo C, Menchetti S, Scordari F (1970) The crystal structure of coquimbite. Atti Accad Naz Lin, Serie 8, 49:129–140
Giester G, Miletich R (1995) Crystal structure and thermal decomposition of the coquimbite-type compound Fe2(SeO4)3·9H2O. Neues Jb Miner Monat 5:211–223
Joeckel RM, Clement BJA, Bates LRVF (2005) Sulfate-mineral crusts from pyrite weathering and acid rock drainage in the Dakota formation and Graneros Shale, Jefferson County, Nebraska. Chem Geol 215:433–452
Libowitzky E, Beran A (2004) IR spectroscopic characterization of hydrous species in minerals. EMU Notes Mineral 6:227–279
Majzlan J, Kiefer B (2006) An X-ray- and neutron-diffraction study of synthetic ferricopiapite, Fe14/3(SO4)6(OD, OH)2(D2O, H2O)20, and ab initio calculations on the structure of magnesiocopiapite, MgFe4(SO4)6(OH)2(H2O)20. Can Mineral 44:1227–1237
Majzlan J, Navrotsky A, McCleskey B, Alpers CN (2006) Thermodynamic properties and crystal structure refinement of ferricopiapite, coquimbite, rhomboclase, and Fe3(SO4)2(H2O)5. Eur J Mineral 18:175–186
Nonius (2001) COLLECT data collection software. Nonius B.V., Delft, The Netherlands
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc Natl Acad Sci USA 96:3455–3462
Otwinowski Z, Borek D, Majewski W, Minor W (2003) Multiparametric scaling of diffraction intensities. Acta Crystallogr A59:228–234
Qin KZ, Ding KS, Xu YX, Miao Y, Fang TH, Xu XW (2008) Tremendous crystal-paracoquimbite and its polytype coquimbite found for the first time in Hongshan Hs-epithermal Cu–Au deposit, eastern Tianshan, NW-China, and its significance. Acta Petrol Sin 24:1112–1122
Rammelsberg CF (1860) Handbuch der Mineralchemie. Verlag von Wilhelm Engelman, Leipzig
Robinson PD, Fang JH (1971) Crystal structures and mineral chemistry of hydrated ferric sulphates: II. The crystal structure of paracoquimbite. Am Mineral 56:1567–1572
Rose H (1833) Ueber einige in Südamerika vorkommende Eisenoxydsalze. Ann Phys 27:309–319
Schefer J, Könnecke M, Murasik A, Czopnik A, Strässle T, Keller P, Schlumpf N (2000) Single-crystal diffraction instrument TriCS at SINQ. Physica B 276–278:168–169
Schulz H (1971) Dispersion analysis of single-crystal diffractometer measurements. II. Variance analysis of a set of intensity measurements. Acta Cryst A27:540–544
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122
Taylor D, Bassett H (1952) The system Al2(SO4)3–H2SO4–H2O. J Chem Soc 4431–4442
Xu W, Tosca NJ, McLennan SM, Parise JB (2009) Humidity-induced phase transitions of ferric sulfate minerals studied by in situ and ex situ X-ray diffraction. Am Mineral 94:1629–1637
Young RA (1996) Introduction to the Rietveld method. IUCr Monogr Crystallogr 5:1–38
Acknowledgments
The neutron diffraction was performed at Trics diffractometer at SINQ, Paul Scherrer Institute (PSI), Villigen, Switzerland. This study was financially supported by the Deutsche Forschungsgemeinschaft, grant MA 3927/2-1. We thank two anonymous reviewers for their constructive criticism and L. Nasdala for the editorial handling of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: J. G. Raith
Rights and permissions
About this article
Cite this article
Majzlan, J., Ðorđević, T., Kolitsch, U. et al. Hydrogen bonding in coquimbite, nominally Fe2(SO4)3·9H2O, and the relationship between coquimbite and paracoquimbite. Miner Petrol 100, 241–248 (2010). https://doi.org/10.1007/s00710-010-0128-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-010-0128-4