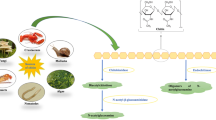
Abstract
Chitinases are a category of hydrolytic enzymes that catalyze chitin and are formed by a wide variety of microorganisms. In nature, microbial chitinases are primarily responsible for chitin decomposition and play a vital role in the balance of carbon and nitrogen ratio in the ecosystem. The physicochemical attributes and the source of chitinase are the main bases that determine their functional characteristics and hydrolyzed products. Several chitinases have been reported and characterized, and they obtain a wider consideration for their utilization in a large number of uses such as in agriculture, food, environment, medicine and pharmaceutical companies. The antifungal and insecticidal impacts of several chitinases have been extensively studied, aiming to protect crops from phytopathogenic fungi and insects. Chitooligosaccharides synthesized by chitin degradation have been shown to improve human health through their antimicrobial, antioxidant, anti-inflammatory and antitumor properties. This review aims at investigating chitinase production, properties and their potential applications in various biotechnological fields.


Similar content being viewed by others
References
Aam BB, Heggset EB, Norberg AL, Sorlie M, Varum KM, Eijsink VG (2010) Production of chitooligosaccharides and their potential applications in medicine. Mar Drugs 8:1482–1517
Abu-Tahon MA, Isaac GS (2020) Anticancer and antifungal efficiencies of purified chitinase produced from Trichoderma viride under submerged fermentation. J Gen Appl Microbiol 66:32–40
Adrangi S, Faramarzi MA (2013) From bacteria to human: a journey into the world of chitinases. Biotechnol Adv 31:1786–1795
Agustiar AA, Faturrohmah I, Sari BW, Isnaini NB, Puspita ID, Husni TA (2019) Increasing chitinase activity of Serratia marcescens PT-6 through optimization of medium composition. Squalen Bull Mar Fish Postharvest Biotech 14(3):113–120
Alhasawi A, Appanna VD (2017) Enhanced extracellular chitinase production in Pseudomonas fluorescens: biotechnological implications. AIMS Bioengin 4(3):366–375
Allonsius CN, Vandenheuvel D, Oerlemans EFM, Petrova MI, Donders GG, Cos P, Delputte P, Lebeer S (2019) Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Sci Rep 9:2900
Al-qwabah AA, Al-limoun MO, Al-Mustafa AH, Al-Zereini WA (2018) Bacillus atrophaeus A7 crude chitinase: characterization and potential role against Drosophila melanogaster larvae. Jord J Biol Sci 11(4):451–459
Alves TB, de Oliveira OPH, de Oliveira AHC, Jorge JA, Guimarães L (2018) Production and characterization of a thermostable antifungal chitinase secreted by the filamentous fungus Aspergillus niveus under submerged fermentation. 3Biotech 8(369): 1-10
Andersen OA, Dixon MJ, Eggleston IM, van Aalten DM (2005) Natural product family 18 chitinase inhibitors. Nat Prod Rep 22(5):563–579
Arai N, Shiomi K, Yamaguchi Y, Masuma R, Iwai Y, Turberg A, Kolbl H, Omura S (2000) Argadin, a new chitinase inhibitor, produced by Clonostachys sp. FO-7314. Chem Pharm Bull (Tokyo) 48(10):1442–1446
Azuma K, Osaki T, Kurozumi S, Kiyose M, Tsuka T, Murahata Y, Imagawa T, Itoh N, Minami S, Sato K, Okamoto Y (2015) Anti-inflammatory effects of orally administered glucosamine oligomer in an experimental model of inflammatory bowel disease. Carbohydr Polym 115:448–456
Bekale L, Agudelo D, Tajmir-Riahi H (2015) Effect of polymer molecular weight on chitosan–protein interaction. Colloids Surf B: Biointerfaces 125:309–317
Benhabiles M, Salah R, Lounici H, Drouiche N, Goosen M, Mameri N (2012) Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll 29:48–56
Berini F, Katz C, Gruzdev N, Casartelli M, Tettamanti G, Marinelli F (2018) Microbial and viral chitinases: attractive biopesticides for integrated pest management. Biotechnol Adv 36:818–838
Berini F, Casartelli M, Montali A, Reguzzoni M, Tettamanti G, Marinelli F (2019) Metagenome-sourced microbial chitinases as potential insecticide proteins. Front Microbiol 10:1–12
Bonanomi G, Lorito M, Vinale F, Woo SL (2018) Organic amendments, beneficial microbes, and soil microbiota: toward a unified framework for disease suppression. Annu Rev Phytopathol 56:1–20
Brzezinska MS, Jankiewicz U, Burkowska A, Walczak M (2014) Chitinolytic microorganisms and their possible application in environmental protection. Curr Microbiol 68:71–81
Burgess L, Knight T, Tesoriero L, VaPhan H (2009) Common diseases of some economically important crops. Australian centre for international agricultural research: Canberra, Australia 11:166–167
Castañeda-Ramírez C, De la Fuente-Salcido NM, Salcedo-Hernández L-GF, Bideshi DK, Barboza-Corona JE (2013) High-level synthesis of endochitinase ChiA74 in Escherichia coli K12 and its promising potential for use in biotechnology. Folia Microbiol 58:455–462
Castillo BM, Dunn MF, Navarro KG, Meléndez FH, Ortiz MH, Guevara SE, Palacios GH (2016) Antifungal performance of extracellular chitinases and culture supernatants of Streptomyces galilaeus CFFSUR-B12 against Mycosphaerella fijiensis Morelet. World J Microbiol Biotechnol 32:44–60
Consortium U (2018) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47:D506–D515
De las Mercedes DM, Pintor-Toro JA, Cubero B (2006) Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol 142: 722–730
De Tender C, Mesuere B, Van der Jeugt F, Haegeman A, Ruttink T, Vandecasteele B, Dawyndt P, Debode J, Kuramae EE (2019) Peat substrate amended with chitin modulates the N-cycle, siderophore and chitinase responses in the lettuce rhizobiome. Sci Rep 9:1–11
Deng JJ, Shi D, Mao HH, Li ZW, Liang S, Ke Y, Luo XC (2019) Heterologous expression and characterization of an antifungal chitinase (Chit46) from Trichoderma harzianum GIM 3.442 and its application in colloidal chitin conversion. Int J Biol Macromol 134:113–121
Djenane Z, Nateche F, Amziane M, Gomis-Cebolla J, El-Aichar F, Khorf H et al (2017) Assessment of the antimicrobial activity and the entomocidal potential of Bacillus thuringiensis isolates from Algeria. Toxins 9:139–155
Dun Y, Li Y, Xu J, Hu Y, Zhang C, Liang Y, Zhao S (2019) Simultaneous fermentation and hydrolysis to extract chitin from crayfish shell waste. Int J Biol Macromol 123:420–426
Egan AM, Sweeney T, Hayes M, O’Doherty JV (2015) Prawn shell chitosan has anti-obesogenic properties, influencing both nutrient digestibility and microbial populations in a pig model. PLoS One 10:e0144127
El-Shora HM, Khalaf SA, El-Sheshtawi SAH (2017) Biochemical characteristics of immobilized Chitinase from Alternaria infectoria. Microbiol Res J Int 22(1):1–10
El-Shora HM, El-Sharkawy RM (2020) Chitinase from Bacillus subtilis: immobilization, antifungal activity and production of N-acetyl-Dglucosamine. Biosci Res 17(1):123–135
Esawy MA, Ghada EA, Awad WA, Abdel Wahab MM, Elnashar A , El-Diwany SH (2016) Immobilization of halophilic Aspergillus awamori EM66 exochitinase on grafted k-carrageenan-alginate beads. 3 biotech 6: 29
Fahmy A, Hassanein R, Hashem H, Ibrahim A, El Shihy O, Qaid E (2018) Developing of transgenic wheat cultivars for improved disease resistance. J Appl Biol Biotechnol 6:31–40
Farag AM, Abd-Elnaby HM, Ibrahim HA, El-Shenawy M (2016) Purification, characterization and antimicrobial activity of chitinase from marine-derived Aspergillus terreus. Egypt J Aqu Res 42:185–192
Gangwar M, Singh V, Pandey AK, Tripathi CKM, Mishra BN (2016) Purification and characterization of chitinase from Streptomyces violascens NRRL B2700. Ind J Exp Biol 54:64–71
Gao L, Sun J, Secundo F, Gao X, Xue C, Mao X (2018) Cloning, characterization and substrate degradation mode of a novel chitinase from Streptomyces albolongus ATCC 27414. Food Chem 261:329–336
Goldman DL, Vicencio AG (2012) The chitin connection. mBio 3(2):e00056-12
Gomaa EZ (2012) Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: their potential in antifungal biocontrol. J Microbiol 50:103–111
Gomaa EZ, El-Mahdy OM (2018) Improvement of chitinase production by Bacillus thuringiensis NM101-19 for antifungal biocontrol through physical mutation. Microbiol 87(4):472–485
Guan GP, Wang HB, Peng HH, Li GY (2016) Low dosage of chitosan supplementation improves intestinal permeability and impairs barrier function in mice Biomed Res Int No:4847296
Guo X, Bai L, Su C, Shi L, Wang D (2013) Molecular cloning and expression of drought-induced protein 3 (dip3) encoding a class III chitinase in upland rice. Gen Mol Res 12:6860–6870
Guo X, Xu P, Zong M, Lou W (2017) Purification and characterization of alkaline chitinase from Paenibacillus pasadenensis CS0611. Chinese J Catal 38:665–672
Halbedel S, Prager R, Banerji S, Kleta S, Trost E, Nishanth G, Alles G, Hölzel C, Schlesiger F, Pietzka A, Schlüter D, Flieger A (2019) A Listeria monocytogenes ST2 clone lacking chitinase ChiB from an outbreak of non-invasive gastroenteritis. Emerg Microbes Infect 8:17–28
Halder SK, Pal S, Mondal KC (2019) Biosynthesis of fungal chitinolytic enzymes and their potent biotechnological appliances. In: Yadav A, Mishra S, Singh S, Gupta A (eds) Recent advancement in white biotechnology through fungi. Springer, Cham, pp 281–298
Han Y, Li ZY, Miao XL, Zhang FL (2008) Statistical optimization of medium components to improve the chitinase activity of Streptomyces sp. Da11 associated with the South China Sea sponge Craniella australiensis. Process Biochem 43:1088–1093
Hirose T (2012) Study on the discovery of novel chitinase inhibitors based on natural products. Yakugaku Zasshi 132(9):1001–1010
Hollensteiner J, Wemheuer F, Harting R, Kolarzyk AM, Diaz Valerio SM, Poehlein A et al (2017) Bacillus thuringiensis and Bacillus weihenstephanensis inhibit the growth of phytopathogenic Verticillium species. Front Microbiol 7:2171
Houston DR, Shiomi K, Arai N, Omura S, Peter MG, Turberg A, Synstad B, Eijsink VG, van Aalten DM (2002) High-resolution structures of a chitinase complexed with natural product cyclopentapeptide inhibitors: mimicry of carbohydrate substrate. Proc Natl Acad Sci U S A 99(14):9127–9132
Huang X, Wang J, Du Z, Zhang C, Li L, Xu Z (2013) Enhanced resistance to stripe rust disease in transgenic wheat expressing the rice chitinase gene RC24. Transgenic Res 22:939–947
Hurtado-Guerrero R, van Aalten DM (2007) Structure of Saccharomyces cerevisiae chitinase 1 and screening-based discovery of potent inhibitors. Chem Biol 14:589–599
Itoh T, Hibi T, Fujii Y, Sugimoto I, Fujiwara A, Suzuki F, Iwasak Y et al (2013) Cooperative degradation of chitin by extracellular and cell surface-expressed chitinases from Paenibacillus sp. strain FPU-7. Appl Environ Microbiol 79:7482–7490
Itoh T, Hibi T, Suzuki F, Sugimoto I, Fujiwara A, Inaka K, et al. (2016) Crystal structure of chitinase ChiW from Paenibacillus sp. str. FPU-7 reveals a novel type of bacterial cell-surface-expressed multi-modular enzyme machinery. PLoS one 11: e0167310
Itoh T, Kimoto H (2019) Bacterial chitinase system as a model of chitin biodegradation. In: Yang Q, Fukamizo T (eds) Targeting chitin-containing organisms. Springer, Singapore, pp 131–151
Jahangiri R, Jamialahmadi K, Behravan J, Fathi Najafi M (2019) Purification and partial characterization of chitinase from a novel strain Aeromonas sp. PTCC 1691. J Mater Environ Sci 10(7):590–597
Jahromi ST, Barzkar N (2018) Marine bacterial chitinase as sources of energy, eco-friendly agent, and industrial biocatalyst. Int J Biol Macromol 120:2147–2154
Jang MK, Kong BG, Jeong YI et al (2004) Physicochemical characterization of alpha-chitin, beta-chitin, and gamma-chitin separated from natural resources. J Polym Sci A Polym Chem 42:3423–3432
Jiang XY, Chen DC, Hong SL, Wang WF, Chen SS, Zou SM (2012) Identification, characterization and functional analysis of a GH-18 chitinase from Streptomyces roseolus. Carbohydr Polym 87:2409–2415
Joao CFC, Silva JC, Borges JP (2015) Chitin-based nanocomposites: biomedical applications. Eco-friendly polymer nanocomposites. Springer, New Delhi, pp 439–457
Juárez-Hernández EO, Casados-Vázquez LE, Del Rincón-Castro MC, Salcedo-Hernández R, Bideshi DK, Barboza-Corona JE (2015) Bacillus thuringiensis subsp. israelensis producing endochitinase ChiA741sp inclusions and its improved activity against Aedes aegypti. J Appl Microbiol 119:1692–1699
Juárez-Hernández EO, Casados-Vázquez LE, Brieba LG, Torres-Larios A, Jimenez-Sandoval P, Barboza-Corona JE (2019) The crystal structure of the chitinase ChiA74 of Bacillus thuringiensis has a multidomain assembly. Sci Rep 9:2591
Jung WJ, Park RD (2014) Bioproduction of chitooligosaccharides: present and perspectives. Mar Drugs 12:5328–5356
Junges Â, Boldo JT, Souza BK, Guedes RL, Sbaraini N, Kmetzsch L, Thompson CE et al (2014) Genomic analyses and transcriptional profiles of the glycoside hydrolase family 18 genes of the entomopathogenic fungus Metarhizium anisopliae. PLoS One 9:e107864
Kaczmarek MB, Struszczyk-Swita K, Li X, Szcze, sna-Antczak M , Daroch M (2019) Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front Bioeng Biotechnol 7: 243
Kawase T, Yokokawa S, Saito A, Fujii T, Nikaidou N, Miyashita K et al (2006) Comparison of enzymatic and antifungal properties between family 18 and 19 chitinases from S. coelicolor A3(2). Biosci Biotechnol Biochem 70:988–998
Kidibule PE, Moriano PS, Ortega EJ, Escudero MR, Limn MC, Remacha M, Plou FJ, Aparicio JS, Lobato MF (2018) Use of chitin and chitosan to produce new chitooligosaccharides by chitinase Chit42: enzymatic activity and structural basis of protein specificity. Microb Cell Factories 17:47
Kong XF, Zhou XL, Lian GQ, Blachier F, Liu G, Tan BE, Nyachoti CM, Yin YL (2014) Dietary supplementation with chitooligosaccharides alters gut microbiota and modifies intestinal luminal metabolites in weaned Huanjiang mini-piglets. Livest Sci 160:97–101
Kuepper M, Bratke K, Virchow JC (2008) Chitinase-like protein and asthma. N Engl J Med 358:1073–1075
Kumar M, Brar A, Vivekanand V, Pareek N (2018) Process optimization, purification and characterization of a novel acidic, thermostable chitinase from Humicola grisea. Int J Biol Macromol 116:931–938
Kumar S, Nehra M, Dilbaghi N, Marrazza G, Hassan AA, Kim KH (2019) Nano-based smart pesticide formulations: emerging opportunities for agriculture. J Control Release 294:131–153
Kusaoke H, Shinya S, Fukamizo T, Kimoto H (2017) Biochemical and biotechnological trends in chitin, chitosan, and related enzymes produced by Paenibacillus IK-5 strain. Int J Biol Macromol 104:1633–1640
Langner T, Öztürk M, Hartmann S, Cord-Landwehr S, Moerschbacher B, Walton JD, Göhre V (2015) Chitinases are essential for cell separation in Ustilago maydis. Eukaryot Cell 14:846–857
Laribi-Habchi H, Bouanane-Darenfed A, Drouiche N, Pauss A, Mameri N (2015) Purification, characterization, and molecular cloning of an extracellular chitinase from Bacillus licheniformis strain LHH100 isolated from wastewater samples in Algeria. Int J Biol Macromol 72:1117–1128
Le B, Yang SH (2018) Characterization of a chitinase from Salinivibrio sp. BAO-1801 as an antifungal activity and a biocatalyst for producing chitobiose. J Basic Microbiol 58:848–856
Le B, Yang SH (2019) Microbial chitinases: properties, current state and biotechnological applications. World J Microbiol Biotechnol 35:144
Lee YG, Chung KC, Wi SG, Lee JC, Bae HJ (2009) Purification and properties of a chitinase from Penicillium sp. LYG 0704. Prot Exp Pur 65:244–250
Lee YS, Kim KY (2015) Statistical optimization of medium components for chitinase production by Pseudomonas fluorescens strain HN1205: role of chitinase on egg hatching inhibition of root-knot nematode. Biotechnol Biotechnol Equip 29(3):470–478
Li XB, Wu PX, Gao GF, Cheng SH (2011) Carbohydrate functionalized chitosan fiber for influenza virus capture. Biomacromol 12:3962–3969
Liang S, Sun Y, Dai X (2018) A review of the preparation, analysis and biological functions of chitooligosaccharide. Int J Mol Sci 19:2197
Liaqat F, Eltem R (2018) Chitooligosaccharides and their biological activities: a comprehensive review. Carbohydr Polym 184:243–259
Liu J, NanGong Z, Zhang J, Song P, Tang Y, Gao Y, Wang Q (2019) Expression and characterization of two chitinases with synergistic effect and antifungal activity from Xenorhabdus nematophila. World J Microbiol Biotechnol 35:105
Liu K, Ding H, Yu Y, Chen B (2019) A cold-adapted chitinase-producing bacterium from Antarctica and its potential in biocontrol of plant pathogenic fungi. Mar Drugs 17:695
Lu Y, Slomberg DL, Schoenfisch MH (2014) Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Biomaterials 35:1716–1724
Lu Y, Wang N, He J, Li Y, Gao X, Huang L, Yan X (2018) Expression and characterization of a novel chitinase with antifungal activity from a rare actinomycete, Saccharothrix yanglingensis Hhs. 015. Protein Exp Pur 143:45–51
Madhuprakash J, Dalhus B, Vaaje-Kolstad G, Sakuda S, Podile AR, Eijsink VG, Sorlie M (2019) Structural and thermodynamic signatures of ligand binding to the enigmatic chitinase D of Serratia proteamaculans. J Phys Chem B 123:2270–2279
Mansour SH, Abdel-Fattah AM, Esawy MA, Ahmed EF, Haroun AA, Hussein MA, Hassanien NM, Khater HM (2019) Immobilization, thermodynamic studies and application of chitinase enzyme from Penicillium chrysogenum. Egypt J Aqu Biol Fish 23(3):527–544
Martínez-Absalón S, Rojas-Solís D, Hernández-León R, Prieto-Barajas C, Orozco-Mosqueda MD, Peña-Cabriales JJ, Sakuda S, Valencia- Cantero E, Santoyo G (2014) Potential use and mode of action of the new strain Bacillus thuringiensis UM96 for the biological control of the grey mould phytopathogen Botrytis cinerea. Biocontrol Sci Tech 24:1349–1362
Mattaveewong T, Wongkrasant P, Chanchai S, Pichyangkura R, Chatsudthipong V, Muanprasat C (2016) Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-κB and mTOR signaling. Carbohydr Polym 145:30–36
Medeiros SC, Monteiro-Júnior JE, Sales GW, Grangeiro TB, Nogueira NA (2018) Chitinases as antibacterial proteins: a systematic review. J Young Pharm 10:144–148
Mei YX, Dai XY, Yang W, Xu XW, Liang YX (2015) Antifungal activity of chitooligosaccharides against the dermatophyte Trichophyton rubrum. Int J Biol Macromol 77:330–335
Miksusanti M, Saputra H, Sandi S, Hermansyah H (2016) The effect of Lactobacillus acidophilus and chito-oligosaccharide on antibacterial activity and organic acid production. Indones J Fundam Appl Chem 1:29–34
Mishra P, Kshirsagar PR, Nilegaonkar SS, Singh SK (2012) Statistical optimization of medium components for production of extracellular chitinase by Basidiobolus ranarum: a novel biocontrol agent against plant pathogenic fungi. J Basic Microbiol 52:539–548
Mohammed AF (2020) Optimization of cellulase and chitinase enzymes production by plant growth promoting Rhizobacteria. Nov Res Microbiol J 4:641–652
Muanprasat C, Chatsudthipong V (2017) Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Ther 170:80–97
Nagpure A, Choudhary B, Gupta RK (2014) Chitinases: in agriculture and human healthcare. Crit Rev Biotechnol 34:215–232
Nasseri A, Rasoul-Amini S, Morowvat M, Ghasemi Y (2011) Single cell protein: production and process. Am J Food Technol 6:103–116
Ohishi K, Murase K, Ohta T, Etoh H (2000) Cloning and sequencing of a chitinase gene from Vibrio alginolyticus H-8. J Biosci Bioeng 89:501–505
Ortiz-Rodríguez T, de la Fuente-Salcido N, Bideshi DK, Salcedo-Hernández R, Barboza-Corona JE (2010) Generation of chitin-derived oligosaccharides toxic to pathogenic bacteria using ChiA74, an endochitinase native to Bacillus thuringiensis. Lett Appl Microbiol 51:184–190
Öztopuz Ö, Pekin G, Park RD, Eltem R (2018) Isolation andevaluation of new antagonist Bacillus strains for the control of pathogenic and mycotoxigenic fungi of fig orchards. Appl Biochem Biotechnol 186:692–711
Pan M, Li J, Lv X, Du G, Liu L (2019) Molecular engineering of chitinase from Bacillus sp. DAU101 for enzymatic production of chitooligosaccharides. Enzym Microb Technol 124:54–62
Pan XQ, Shih CC, Harday J (2005) Chitinase induces lysis of MCF-7 cells in culture and of human breast cancer xenograft B11–2 in SCID mice. Anticancer Res 25:3167–3172
Park JK, Chung MJ, Choi HN, Park YI (2011) Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int J Mol Sci 12:266–277
Patel S, Goyal A (2011) Functional oligosaccharides: production, properties and applications. World J Microbiol Biotechnol 27:1119–1128
Patil NS, Jadhav JP (2014) Enzymatic production of N-acetyl-D-glucosamine by solid state fermentation of chitinase by Penicillium ochrochloron MTCC517 using agricultural residues. Int Biodeterior Biodegrad 91:9–17
Pinnamaneni R, Kalidas P, Rao KS (2010) Cloning and expression of Bbchit1 gene of Beauveria bassiana. The Open Entomol J 4:30–35
Prakash NAU, Jayanthi M, Sabarinathan R et al (2010) Evolution, homology conservation, and identification of unique sequence signatures in GH19 family chitinases. J Mol Evol 70:466–478
Rathore AS, Gupta RD (2015) Chitinases from bacteria to human: properties, applications, and future perspectives. Enzyme Res 791907
Rao FV, Houston DR, Boot RG, Aerts JM, Hodkinson M, Adams DJ, Shiomi K, Omura S, van Aalten DM (2005) Specificity and affinity of natural product cyclopentapeptide inhibitors against A. fumigatus, human, and bacterial chitinases. Chem Biol 12(1):65–76
Revathi M, Saravanan R, Shanmugam A (2012) Production and characterization of chitinase from Vibrio species, a head waste of shrimp Metapenaeus dobsonii (Miers, 1878) and chitin of Sepiella inermis Orbigny, 1848. Adv Biosci Biotech 3:392–397
Richa K, Tiwari IM, Devanna B, Botella JR, Sharma V, Sharma TR (2017) Novel chitinase gene LOC_Os11g47510 from Indica Rice Tetep provides enhanced resistance against sheath blight pathogen Rhizoctonia solani in rice. Front Plant Sci 8:596
Robbins PW, Albright C, Benfield B (1988) Cloning and expression of a Streptomyces plicatus chitinase (chitinase-63) in Escherichia coli. J Biol Chem 263:443–447
Sadfi N, Cherif M, Fliss I, Boudabbous A, Antoun H (2001) Evaluation of bacterial isolates from salty soils and Bacillus thuringiensis strains for the biocontrol of Fusarium dry rot of potato tubers. J Plant Pathol 83:101–117
Selenius O, Korpela J, Salminen S, Gallego CG (2018) Effect of chitin and chitooligosaccharide on in vitro growth of Lactobacillus rhamnosus GG and Escherichia coli TG. Appl Food Biotechnol 5:163–172
Senol M, Nadaroglu H, Dikbas N, Kotan R (2014) Purification of chitinase enzymes from Bacillus subtilis bacteria TV-125, investigation of kinetic properties and antifungal activity against Fusarium culmorum. Annal Clin Microbiol Antimicrobial 13:35
Shahmoradi ZS, Tohidfar M, Marashi H, Malekzadeh-Shafaroudi S, Karimi E (2019) Cultivation effect of Chitinase-transgenic cotton on functional Bacteria and Fungi in Rhizosphere and bulk soil. Iran J Biotech 17(2):55–60
Sharma CK, Vishnoi VK, Dubey R, Maheshwari DA (2018) A twin rhizospheric bacterial consortium induces systemic resistance to a phytopathogen Macrophomina phaseolina in mung bean. Rhizosphere 5:71–75
Sharma R, Singh D, Singh R (2009) Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control 50:205–221
Shin S, Mackintosh CA, Lewis J, Heinen SJ, Radmer L, Dill-Macky R, Baldridge GD, Zeyen RJ, Muehlbauer GJ (2008) Transgenic wheat expressing a barley class ii chitinase gene has enhanced resistance against Fusarium graminearum. J Exp Bot 59:2371–2378
Shrestha A, Sultana R, Chae JC, Kim K, Lee KJ (2015) Bacillus thuringiensis C25 which is rich in cell wall degrading enzymes efficiently controls lettuce drop caused by Sclerotinia minor. Eur J Plant Pathol 142:577–589
Singh AK, Mehta G, Chhatpar HS (2009) Optimization of medium constituents for improved chitinase production by Paenibacillus sp. D1 using statistical approach. Lett Appl Microbiol 49:708–714
Singh HR, Deka M, Das S (2015) Enhanced resistance to blister blight in transgenic tea (Camellia sinensis [L.] o. Kuntze) by overexpression of class I chitinase gene from potato (Solanum tuberosum). Funct Integr Genom 15:461–480
Sousa AJ, Silva FB, Sousa JS, Júnior JE, Freire JE, Sousa BL et al (2019) A thermostable chitinase from the antagonistic Chromobacterium violaceum that inhibits the development of phytopathogenic fungi. Enzym Microb Technol 126:50–61
Srinivasan H, Kanayairam V, Ravichandran R (2018) Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int J Biol Macromol 107:662–667
Stumpf AK, Vortmann M, Dirks-Hofmeister M, Moerschbacher B, Philipp B (2019) Identification of a novel chitinase from Aeromonas hydrophila AH-1N for the degradation of chitin within fungal mycelium. FEMS Microbiol Lett 366(1):1–15
Suganthi M, Senthilkumar P, Arvinth S, Chandrashekara KN (2017) Chitinase from Pseudomonas fluorescens and its insecticidal activity against Helopeltis theivora. J Gen Appl Microbiol 63:222–227
Suginta W, Sirimontree P, Sritho N, Ohnuma T, Fukamizo T (2016) The chitin binding domain of a GH-18 Chitinase from Vibrio harveyi is crucial for chitin-chitinase interactions. Int J Biol Macromol 93:1111–1117
Sukalkar SR, Kadam TA, Bhosale HJ (2018) Optimization of chitinase production from Streptomyces macrosporeus M1. RJLBPCS 4(1):106–114
Sun X, Li Y, Tian Z, Qian Y, Zhang H, Wang L (2019) A novel thermostable chitinolytic machinery of Streptomyces sp. F-3 consisting of chitinases with different action modes. Biotechnol Biofuel 12:136–148
Suresh P, Kumar PA (2012) Enhanced degradation of α-chitin materials prepared from shrimp processing byproduct and production of N-acetyl-D-glucosamine by thermoactive chitinases from soil mesophilic fungi. Biodegradation 23:597–607
Take K, Fujiki H, Suyotha W, Hayashi J, Takagi K, Yano S, Wakayama M (2018) Enzymatic and molecular characterization of an acidic and thermostable chitinase 1 from Streptomyces thermodiastaticus HF 3–3. J gen Appl Microbiol. Https ://doi.org/10.2323/jgam.2017.12.002
Tang Y, Zou J, Zhang L, Li Z, Ma C, Ma N (2012) Anti-fungi activities of Bacillus thuringiensis H3 chitinase and immobilized chitinase particles and their effects to rice seedling defensive enzymes. J Nanosci Nanotechnol 12:8081–8086
Tao A, Pang F, Huang S, Yu G, Li B, Wang T (2014) Characterization of endophytic Bacillus thuringiensis strains isolated from wheat plants as biocontrol agents against wheat flag smut. Biocontrol Sci Tech 24:901–924
Tom RA, Carroad PA (1981) Effect of reaction conditions on hydrolysis of chitin by Serratia marcescens QMB 1466 chitinase. J Food Sci 46:646–647
Udaya NA, Jayanthi M, Sabarinathan R, Kangueane P, Mathew L, Sekar K (2010) Evolution, homology conservation, and identification of unique sequence signatures in GH19 family chitinases. J Mol Evol 70:466–478
Vaaje-Kolstad G, Horn SJ, Sørlie M, Eijsink VG (2013) The chitinolytic machinery of Serratia marcescens - a model system for enzymatic degradation of recalcitrant polysaccharides. FEBS J 280:3028–3049
Vahed M, Motalebi E, Rigi G, Noghabi KA, Soudi MR, Sadeghi M et al (2013) Improving the chitinolytic activity of Bacillus pumilus SG2 by random mutagenesis. J Microbiol Biotechnol 23:1519–1528
Vaidya R, Vyas P, Chhatpar HS (2003) Statistical optimization of medium components for the production of chitinase by Alcaligenes xylosoxydans. Enzyme Microbial Technol 33:92–96
Vetter J (2007) Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Food Chem 102:6–9
Vyas PR, Deshpande MV (1991) Enzymatic hydrolysis of chitin by. Myrothecium verrucariachitinase complex and its utilization to produce SCP J Gen Appl Microbiol 37(3):267–275
Vyawahare MP, Ingle ST, Gathe AG (2019) Morphological variation and chitinase production ability of. Trichoderma viride mutants Int J Curr Microbiol Appl Sci 8(1):447–455
Wan J, Jiang F, Xu QS, Chen DW, Yu B, Huang ZQ, Mao X, Yu J, He J (2017) New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv 7:9669–9679
Watanabe T, Kobori K, Miyashita K, Fujii T, Sakai H, Uchida M, Tanaka H (1993) Identification of glutamic acid 204 and aspartic acid 200 in chitinase A1 of Bacillus circulans WL-12 as essential residues for chitinase activity. J Biol Chem 268:18567–18572
Watanabe T, Kanai R, Kawase T, Tanabe T, Mitsutomi M, Sakuda S, Miyashita K (1999) Family 19 chitinases of Streptomyces species: characterization and distribution. Microbiology 145:3353–3363
Webb DC, McKenzie AN, Foster PS (2001) Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J Biol Chem 276:41969–41976
Wu AC, Lasky-Su J, Rogers CA, Klanderman BJ, Litonjua A (2010) Polymorphisms of chitinases are not associated with asthma. J Allergy Clin Immunol 125:754–757
Xiao DF, Ren WK, Bin P, Chen S, Yin J, Gao W, Liu G, Nan Z, Hu X, He J (2016) Chitosan lowers body weight through intestinal microbiota and reduces IL-7 expression via mTOR signalling. J Funct Foods 22:166–176
Yadav M, Goswami P, Paritosh K, Kumar M, Pareek N, Vivekanand V (2019) Seafood waste: a source for preparation of commercially employable chitin/chitosan materials. Biores Biopros 6:8
Yan Q, Fong SS (2018) Cloning and characterization of a chitinase from Thermobifida fusca reveals Tfu_0580 as a thermostable and acidic endochitinase. Biotechnol Rep 19:e00274
Yan L, Kim IH (2011) Evaluation of dietary supplementation of deltaaminolevulinic acid and chitooligosaccharide on growth performance, nutrient digestibility, blood characteristics, and fecal microbial shedding in weaned pigs. Anim Feed Sci Tech 169:275–280
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13:1133–1174
Zhang P, Liu WZ, Peng YF, Han BQ, Yang Y (2014) Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages. Int Immunopharmacol 23:254–261
Zhang A, He Y, Wei G, Zhou J, Dong W, Chen K, Ouyang P (2018) Molecular characterization of a novel chitinase cm Chi1 from Chitinolyticbacter meiyuanensis SYBC-H1 and its use in N-acetyl-d-glucosamine production. Biotechnol Biofuels 11:179
Zhou J, Chen L, Kang L, Liu Z, Bai Y, Yang Y, Yuan S (2018) ChiE1from Coprinopsis cinerea is characterized as a processive exochitinase and revealed to have a significant synergistic action with endochitinase ChiIII on chitin degradation. J Agric Food Chem 66:12773–12782
Zong H, Li K, Liu S, Song L, Xing R, Chen X, Li P (2017) Improvement in cadmium tolerance of edible rape (Brassica rapa L.) with exogenous application of chitooligosaccharide. Chemosphere 181:92–100
Zou P, Li K, Liu S, He X, Zhang X, Xing R, Li P (2016) Effect of sulfated chitooligosaccharides on wheat seedlings (Triticum aestivum L.) under salt stress. J Agric Food Chem 64:2815–2821
Zou P, Yuan S, Yang X, Zhai X, Wang J (2018) Chitosan oligosaccharides with degree of polymerization 2–6 induces apoptosis in human colon carcinoma HCT116 cells. Chem Biol Interact 279:129–135
Author information
Authors and Affiliations
Contributions
Eman Zakaria Gomaa has established the main idea, compiled the literature and revised the review.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the author.
Additional information
Handling Editor: Handling Editor: Klaudia Brix
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomaa, E.Z. Microbial chitinases: properties, enhancement and potential applications. Protoplasma 258, 695–710 (2021). https://doi.org/10.1007/s00709-021-01612-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01612-6