Abstract
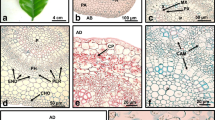
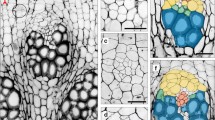
Development of cambium and its activity is important for our knowledge of the mechanism of secondary growth. Arabidopsis thaliana emerges as a good model plant for such a kind of study. Thus, this paper reports on cellular events taking place in the interfascicular regions of inflorescence stems of A. thaliana, leading to the development of interfascicular cambium from differentiated interfascicular parenchyma cells (IPC). These events are as follows: appearance of auxin accumulation, PIN1 gene expression, polar PIN1 protein localization in the basal plasma membrane and periclinal divisions. Distribution of auxin was observed to be higher in differentiating into cambium parenchyma cells compared to cells within the pith and cortex. Expression of PIN1 in IPC was always preceded by auxin accumulation. Basal localization of PIN1 was already established in the cells prior to their periclinal division. These cellular events initiated within parenchyma cells adjacent to the vascular bundles and successively extended from that point towards the middle region of the interfascicular area, located between neighboring vascular bundles. The final consequence of which was the closure of the cambial ring within the stem. Changes in the chemical composition of IPC walls were also detected and included changes of pectic epitopes, xyloglucans (XG) and extensins rich in hydroxyproline (HRGPs). In summary, results presented in this paper describe interfascicular cambium ontogenesis in terms of successive cellular events in the interfascicular regions of inflorescence stems of Arabidopsis.









Similar content being viewed by others
References
Agusti J, Greb T (2013) Going with the wind – adaptive dynamics of plant secondary meristems. Mech Dev 130:34–44
Agusti J, Lichtenberger R, Schwarz M, Nehlin L, Greb T (2011a) Characterization of transcriptome remodeling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth. PLoS Genet 7:e1001312. doi:10.1371/journal.pgen.1001312
Agusti J, Herold S, Schwartz M, Sanchez P, Ljung K, Dun EA, Brewer PB, Beveridge CA, Sieberer T, Sehr E, Greb T (2011b) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. PNAS 7:1–6
Aloni R, Zimmermann MH (1983) The control of vessel size and density along the plant axis – a new hypothesis. Differentiation 24:203–208
Altamura MM, Possenti M, Matteucci A, Baima S, Ruberti I, Morelli G (2001) Development of the vascular system in the inflorescence stem of Arabidopsis. New Phytol 151:81–389
Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S (2011) Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J 65:571–577
Benitez-Alfonso Y, Faulkner C, Miyashima S, Helariutta Y, Maule A (2013) Symplastic intercellular connectivity regulates lateral root patterning. Dev Cell 26:136–147
Benkova E, Ivanchenko MG, Friml J, Shishkova S, Dubrovsky JG (2009) A morphogenetic trigger: is there an emerging concept in plant developmental biology? Trends Plant Sci 14:189–193
Bhalerao RP, Bennett MJ (2003) The case for morphogens in plants. Nat Cell Biol 5:939–943
Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J, Geisler M, Nagashima A, Sakai T, Martinoia E, Friml J, Peer WA, Murphy AS (2007) Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19:131–147
Busse JS, Evert RF (1999) Vascular differentiation and transition in the seedling of Arabidopsis thaliana (Brassicaceae). Int J Plant Sci 160:41–251
Campanoni P, Nick P (2005) Auxin-dependent cell division and cell elongation. 1-Naphtaleneacetic acid and 2,4-dichlorophenoxyacetic acid activate different pathways. Plant Physiol 137:939–948
Carpita N, Tierney M, Campbell M (2001) Molecular biology of the plant cell wall: searching for the genes that define structure, architecture and dynamics. Plant Mol Biol 47:1–5
Chaffey N (1999) Cambium: old challenges – new opportunities. Trees 13:138–151
Chaffey N (2002) Why is there so little research into the cell biology of the secondary vascular system of trees? New Phytol 153:213–223
Chaffey N, Cholewa E, Regan S, Sundberg B (2002) Secondary xylem development in Arabidopsis: a model for wood formation. Physiol Plant 114:594–600
Chen JG (2001) Dual auxin signaling pathways control cell elongation and division. J Plant Growth Regul 20:255–264
Darley CP, Forrester AM, McQueen-Mason SJ (2001) The molecular basis of plant cell wall extension. Plant Mol Biol 47:179–195
Dettmer J, Elo A, Helariuta Y (2008) Hormone interactions during vascular development. Plant Mol Biol. doi:10.1007/s11103-008-9374-9
Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119:71–84
Dubois T, Guedira M, Dubois J, Vasseur J (1990) Direct somatic embryogenesis in roots of Cichorium: is callose an early marker? Ann Bot 65:539–545
Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benkova E (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. PNAS 105:8790–8794
Evert RF (2006) Esau’s plant anatomy: meristems, cells, and tissues of the structure, function, and development, 3rd edn. Wiley, New Jersey
Friml J (2010) Subcellular trafficking of PIN auxin carriers in auxin transport. EJCB 89:231–235
Friml J, Wiśniewska J (2005) Auxin as an intercellular signal. Intercellular communication in plants. In: Fleming A (ed), Blackwell Publishing Limited, pp 1–25
Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish apical–basal axis of Arabidopsis. Nature 426:147–153
Fukuda H (1997) Tracheary element differentiation. Plant Cell 9:1147–1156
Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, Ejendal KF, Smith AP, Baroux C, Grossniklaus U, Müller A, Hrycyna CA, Dudler R, Murphy AS, Martinoia E (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44:179–194
Gonneau M, Höfte H, Vernhettes S (2012) Fluorescent tags to explore cell wall structure and dynamics. Front Plant Sci 3:1–6
Gray-Mitsumine M, Mellerowicz EJ, Abe H, Schrader J, Winzell A, Sterky F, Blomqvist K, McQueen-Mason S, Teeri TT, Sundberg B (2004) Expansins abundant in secondary xylem belong to subgroup A of the α-expansin gene family. Plant Physiol 135:1552–1564
Hayashi T, Kaida R (2011) Functions of xyloglucan in plant cells. Mol Plant 4:17–24
Hellgren JM, Olofsson K, Sundberg B (2004) Patterns of auxin distribution during gravitational induction of reaction wood in poplar and pine. Plant Physiol 135:212–220
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: ß-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jose-Estanyol M, Puigdomenech P (2000) Plant cell wall glycoproteins and their genes. Plant Physiol Biochem 38:97–108
Kitakura S, Vanneste S, Robert S, Löfke C, Teichmann T, Tanaka H, Friml J (2011) Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell 23:1920–1931
Knox JP (1997) The use of antibodies to study the architecture and developmental regulation of plant cell walls. Int Rev Cytol 171:79–120
Ko JH, Han KH (2004) Arabidopsis whole-transcriptome profiling defines the features of coordinated regulations that occur during secondary growth. Plant Mol Biol 55:433–453
Ko JH, Han KH, Park S, Yang J (2004) Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiol 135:1069–1083
Kramer EM (2006) Wood grain pattern formation: a brief review. J Plant Growth Regul 25:290–301
Kurczyńska EU, Gaj MD, Ujczak A, Mazur E (2007) Histological analysis of direct somatic embryogenesis in Arabidopsis thaliana (L.) Heynh. Planta 226:619–628
Larson PR (1994) The vascular cambium. Development and structure. Springer, Berlin
Lev-Yadun S (1994) Induction of sclereid differentiation in the pith of Arabidopsis thaliana (L.) Heynh. J Exp Bot 45:1845–1849
Lev-Yadun S (1997) Fibres and fibre-sclereids in wild-type Arabidopsis thaliana. Ann Bot 80:125–129
Lev-Yadun S, Flaishman MA (2001) The effect of submergence on ontogeny of cambium and secondary xylem and on fiber lignification in inflorescence stems of Arabidopsis. IAWA J 22:159–169
Little CHA, Wareing PF (1981) Control of cambial activity and dormancy in Picea sitchensis by indol-3-ylacetic and abscisic acids. Can J Bot 59:1480–1493
Little CHA, MacDonald JE, Olsson O (2002) Involvement of indole-3-acetic acid in fascicular and interfascicular cambial growth and interfascicular extraxylary fiber differentiation in Arabidopsis thaliana inflorescence stems. Int J Sci 163:519–529
Mazur E, Kurczynska EU (2012) Rays, intrusive growth and storied cambium in Arabidopsis thaliana. Protoplasma 249:217–220
Mellerowicz EJ, Baucher M, Sundberg B, Boerjan W (2001) Unravelling cell wall formation in the woody dicot stem. Plant Mol Biol 47:239–274
Nieminen KM, Kauppinen L, Helariutta Y (2004) A weed for wood? Arabidopsis as a genetic model for xylem development. Plant Physiol 135:653–659
Nishikubo N, Takahashi J, Roos AA, Derba-Maceluch M, Piens K, Brumer H, Teeri TT, Stälbrand H, Mellerowicz EJ (2011) Xyloglucan endo-transglycosylase-mediated xyloglucan rearrangement in developing wood of hybrid aspen. Plant Physiol 155:399–413
O’Brien TP, McCully ME (1981) The study of plant structure. Principles and selected methods. Termarcarphi Pty Ltd, Melbourne
Oh S, Park S, Han KH (2003) Transcriptional regulation of secondary growth in Arabidopsis thaliana. J Exp Bot 393:2709–2722
Paciorek T, Sauer M, Balla J, Wiśniewska J, Friml J (2006) Immunocytochemical technique for protein localization in sections of plant tissues. Nat Protoc 1:104–107
Paul-Victor C, Rowe N (2011) Effect of mechanical perturbation on the biomechanics, primary growth and secondary tissue development of inflorescence stems of Arabidopsis thaliana. Ann Bot 107:209–218
Pedroso MC, Pais MS (1992) A scanning electron microscope and x-ray microanalysis study during induction of morphogenesis in Camellia japonica L. Plant Sci 87:99–108
Petrašek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wiśniewska J, Tadele Z, Kubeš M, Čovanova M, Dhonukshe P, Skupa P, Benkova E, Perry L, Křecek P, Lee OR, Fink GR, Giesler M, Murphy AS, Luschnig C, Zažimalova E, Friml J (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312:914–918
Philipson WR, Ward JM, Butterfield BG (1971) The vascular cambium. Its development and activity. Chapman and Hall, London
Romberger JA, Hejnowicz Z, Hill JF (1993) The vascular cambium. Plant structure: function and development. Springer, Berlin, pp 354–385
Sachs T (1975) The induction of transport channels by auxin. Planta 127:201–206
Sachs T (1981) The control of the patterned differentiation of vascular tissues. Adv Bot Res 9:151–262
Sauer M, Balla J, Luschnig C, Wiśniewska J, Reinöhl V, Friml J, Benkova E (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20:2902–2911
Schrader J, Baba K, May ST, Palme K, Bennett M, Bhalerao RP, Sandberg G (2003) Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of development and environmental signals. Proc Natl Acad Sci 100:10096–10101
Sehr EM, Agusti J, Lehner R, Farmer EE, Schwartz M, Greb T (2010) Analysis of secondary growth in Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J 63:811–822
Suer S, Agusti J, Sanchez P, Schwartz M, Greb T (2011) WOX4 imparts auxin responsiveness to cambium cells in Arabidopsis. Plant Cell 23:3247–3259
Sundberg B, Uggla C, Tuominen H (2000) Auxin gradients and cambial growth. In: Savidge R, Barnett J, Napier R (eds) Cell and molecular biology of wood formation (SEB Experimental Biology Reviews). BIOS, Oxford, pp 169–188
Takeda T, Furuta Y, Awano K, Mitsuishi Y, Hayashi T (2002) Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. PNAS 99:9055–9060
Tchorbadjieva MI (2005) Protein markers for somatic embryogenesis. Plant Cell Monogr (2). In: Mujib A, Šamaj J (eds) Somatic embryogenesis. Springer, Berlin, pp 215–233
Tuominen H, Puech L, Regan S, Fink S, Olsson O, Sundberg B (2000) Cambial-region-specific expression of the Agrobacterium iaa genes in transgenic aspen visualized by a linked uidA reporter gene. Plant Physiol 123:531–542
Uggla C, Moritz T, Sandberg G, Sundberg B (1996) Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci 93:9282–9296
Uggla C, Mellerowicz EJ, Sundberg B (1998) Indole-3-acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiol 117:113–121
Ulmasov T, Murfett J, Hagen G, Gulifoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Vatén A, Dettmer J, Wu S, Stierhof YD, Miyashima S, Yadav SR, Roberts CJ, Campilho A, Bulone V, Lichtenberger R, Lehesranta S, Mahönen AP, Kim JY, Jokitalo E, Sauer N, Scheres B, Nakajima K, Carlsbecker A, Gallangher KL, Helariutta Y (2011) Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell 21:1144–1155
Vieten A, Vanneste S, Wiśniewska J, Benkova E, Benjamins R, Beeckman T, Luschnig C, Friml J (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132:4521–4531
Wabnik K, Kleine-Vehn J, Balla J, Sauer M, Naramoto S, Reinöhl V, Merks RMH, Govaerts W, Friml J (2010) Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling. Mol Syst Biol 6:1–15
Wang X, Sager R, Cui W, Zhang C, Lee JY (2013) Salicylic acid regulates plasmodesmata closure during innate immune responses in Arabidopsis. Plant Cell. doi:10.1105/tpc.113.110676
Willats WGT, Steele-King CG, McCartney L, Orfila C, Marcus SE, Knox JP (2000) Making and using antibody probes to study plant cell walls. Plant Physiol Biochem 38:27–36
Ye ZH, Varner JE (1991) Tissue-Specific Expression of Cell Wall Proteins in Developing Soybean Tissues. Plant Cell 3:23–37
Ye ZH, Song YR, Marcus A, Varner JE (1991) Comparative localization of three classes of cell wall proteins. Plant J 1:175–183
Zhao C, Johnson BJ, Kositsup B, Beers EP (2000) Exploiting secondary growth in Arabidopsis. Construction of xylem and bark cDNA libraries and cloning of three xylem endopeptidases. Plant Physiol 123:1185–1196
Acknowledgments
We express our gratitude to Professor Paul Knox for the anti-extensin LM1 antibody probe for HRGPs detection and Dr. Timothy C. Baldwin (University of Wolverhampton) for his assistance in the preparation of the English version of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Alexander Schulz
Rights and permissions
About this article
Cite this article
Mazur, E., Kurczyńska, E.U. & Friml, J. Cellular events during interfascicular cambium ontogenesis in inflorescence stems of Arabidopsis . Protoplasma 251, 1125–1139 (2014). https://doi.org/10.1007/s00709-014-0620-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0620-5