Abstract
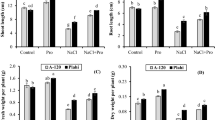
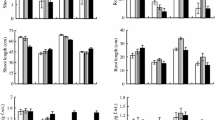
The present study suggests the involvement of proline in copper tolerance of four genotypes of Cicer arietinum (chickpea). Based on the data of tolerance index and lipid peroxidation, the order for copper tolerance was as follows: RSG 888 > CSG 144 > CSG 104 > RSG 44 in the selected genotypes. The basis of differential copper tolerance in chickpea genotypes was characterized by analyzing, antioxidant enzymes (superoxide dismutase, ascorbated peroxidase and catalase), phytochelatins, copper uptake, and proline accumulation. Chickpea genotypes showed stimulated superoxide dismutase activity at all tested concentrations of copper, but H2O2 decomposing enzymes especially; ascorbate peroxidase did not increase with 25 and 50 μM copper treatments. Catalase activity, however, increased at lower copper concentrations but failed to stimulate at 50 μM copper. Such divergence in responses of these enzymes minimizes their importance in protecting chickpea against copper stress. The sensitive genotypes showed greater enhancement of phytochelatins than that of tolerant genotypes. Hence, the possibility of phytochelatins in improving copper tolerance in the test plant is also excluded. Interestingly, the order of proline accumulation in the chickpea genotypes (RSG 888 > CSG 144 > CSG 104 > RSG 44) was exactly similar to the order of copper tolerance. Based on hyperaccumulation of proline in tolerant genotype (RSG 44) and the reduction and improvement of lipid peroxidation and tolerance index, respectively, by proline pretreatment, we conclude that hyperaccumulation of proline improves the copper tolerance in chickpea.



Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- BSO:
-
l-buthionine-[S,R]-sulphoximine
- CAT:
-
catalase
- GSH:
-
reduced glutathione
- GSSG:
-
oxidized glutathione
- MDA:
-
malondialdehyde
- SOD:
-
superoxide dismutase
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alia, Prasad KVSK, Paradha Saradhi P (1995) Effect of zinc on free radicals and proline in Brassica and Cajanus. Phytochem 39:45–47
Alia, Mohanty P, Matysik J (2001) Effect of proline on the production of singlet oxygen. Amino Acids 21:195–200
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Asada K (1984) Chloroplasts: formation of active oxygen and its scavenging. Meth Enzymol 105:422–435
Bates LS, Waldren RP, Tear ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bates SS, Tessier A, Campbell PGC, Buffle J (1982) Zinc adsorption and transport by Chlamydomonas variabilis and Scenedesmus subspicatus (Chlorophyceae) grown in semicontinuous culture. J Phycol 18:521–529
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44:273–287
Burkhead JL, Reynolds KAG, Abdel-Ghany SE, Cohu CM, Pilon M (2009) Copper homeostasis. New Phytol 182:799–816
Clairbone A (1985) Catalase activity. In: Greewald RA (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 283–284
Claussen W (2005) Proline as a measure of stress in tomato plants. Plant Sci 168:241–248
Culotta V (2010) Cell biology of copper. J Biol Inorg Chem 15:1–2
De Vos CHR, Schat H, Vooijs R, Ernst WHO (1989) Copper-induced damage to the permeability barrier in roots of Silene cucubalus. J Plant Physiol 135:164–179
De Vos CHR, Vonk MJ, Vooijs R, Schat H (1992) Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 98:853–858
Dietz KJ, Baier M, Kramer U (1999) Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In: Prasad MNV (ed) Heavy metal stress in plants: from molecules to ecosystems. Springer, Berlin, pp 73–79
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek S, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma (In Press). doi:10.1007/s00709-010-0144-6
Knauert S, Kanuer K (2008) The role of reactive oxygen species in copper toxicity to two freshwater green algae. J Phycol 44:311–319
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61:517–534
Kwon TW, Menzel DB, Olcott HS (1965) Reactivity of malondialdehyde with food constituents. J Food Sci 30:808–813
Lowry OH, Rosenbrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luna CM, Gonzalez CA, Trippi VS (1994) Oxidative damage caused by excess copper in oat leaves. Plant Cell Physiol 35:11–15
Maksymiec W (1997) Effect of Cu on cellular processes in higher plants. Photosynthetica 34(3):321–342
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Martins LL, Mourato MP (2006) Effect of excess copper on tomato plants: growth parameters, enzyme activities, chlorophyll, and mineral content. J Plant Nutr 29:2179–2198
Matysik J, Alia BB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Mehta SK, Gaur JP (1999) Heavy metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol 143:253–259
Metwally A, Safronova VI, Belimov AA, Dietz KJ (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativum. J Exp Bot 56:167–178
Schat H, Llugany M, Vooij R, Hartley-Whitaker J, Bleeker PM (2002) The role of phytochelatins in constitutive and adaptive heavy metal tolerance in hyperaccumulator and non-hyperaccumulator metallophytes. J Exp Bot 53:2381–2392
Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorhization. J Exp Bot 53:1351–1365
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Siripornadulsil S, Traina S, Verma DPS, Sayre RT (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14:2837–2847
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochem 28:1057–1060
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol Med 18:321–336
Tripathi BN, Gaur JP (2004) Relationship between copper and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 219:397–404
Tripathi BN, Gaur JP (2006) Physiological behaviour of Scenedesmus sp. during exposure to elevated levels of Cu and Zn, and after withdrawing metal stress. Protoplasma 229:1–9
Tripathi BN, Mehta SK, Amar A, Gaur JP (2006) Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu and Zn. Chemosphere 62:538–544
Tripathi BN, Bhatt I, Dietz KJ (2009) Peroxiredoxins: a less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma 235:3–15
Tsuji N, Hirayanagi N, Okada M, Miyasaka H, Hirata K, Zenk MH, Miyamoto K (2002) Enhancement of tolerance to heavy metals and oxidative stress in Dunaliella tertiolecta by Zn-induced phytochelatin synthesis. Biochem Biophys Res Comm 293:653–659
Van Assche F, Clijsters HC (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Vinit-Dunand F, Epron D, Alaoui-Sosse B, Badot P-M (2002) Effects of copper on growth and on photosynthesis of mature and expanding leaves in cucumber plants. Plant Sci 163:53–58
Wu JT, Chang SC, Chen KS (1995) Enhancement of intracellular proline levels in cells of Anacystis nidulans (cyanobacteria) exposed to deleterious concentrations of copper. J Phycol 31:376–379
Xu J, Yin HX, Li X (2009) Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep 28:325–333
Acknowledgment
We are thankful to Professor Aditya Shastri, Vice-Chancellor of the Banasthali University for necessary facilities. B.N.T. thanks Department of Science and Technology, New Delhi, for financial support.
Conflict of interest
Authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, V., Bhatt, I., Aggarwal, A. et al. Proline improves copper tolerance in chickpea (Cicer arietinum). Protoplasma 245, 173–181 (2010). https://doi.org/10.1007/s00709-010-0178-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0178-9