Abstract
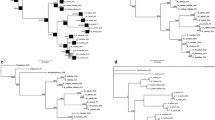
The internal transcribed spacers (ITS) of nuclear ribosomal DNA (nrDNA) and plastid DNA, including the trnL intron, the trnL-F spacer, and the atpB-rbcL spacer, from most of the living species in the genus Phalaenopsis were sequenced. The monophyly of the genus described by Christenson (Christenson EA (2001) Phalaenopsis. Timber Press, Portland, p 330), that Doritis and Kingidium are synonyms of Phalaenopsis, was supported by these molecular data. Within the genus, subgenus Polychilos was monophyletic, and the species were divided into two subclades. The subgenus Phalaenopsis was shown to be non-monophyletic, because the sections Esmeralda and Deliciosae appeared separated the from sections Phalaenopsis and Stauroglottis. Meanwhile, subgenera Aphyllae and Parishianae were also shown to be non-monophyletic on the basis of the molecular data. Furthermore, the monotypic species of subgenus Proboscidioides, P. lowii, formed a clade with subgenus Aphyllae. In accordance with geographical distribution, the historical geography of Southeast Asia due to the periodic glacial epochs, and molecular phylogeny, two evolutionary trends of Phalaenopsis from the original center in South China to the Philippines, Indonesia, and Malaysia were suggested. First, using Indochina and some older parts of the Philippines (e.g., Mindoro and Palawan) as stepping stones, Phalaenopsis species dispersed from South China to the Philippines, where the sections Phalaenopsis and Stauroglottis of subgenus Phalaenopsis developed. Second, using the Malay Peninsula as a stepping stone, Phalaenopsis species dispersed from South China to Indonesia and Malaysia, where the subgenus Polychilos developed.









Similar content being viewed by others
References
Aurelio MA, Barrier E, Rangin C, Muller C (1991) The Philippine Fault in the late Cenozoic tectonic evolution of the Bondoc–Masbate–N. Leyte area, central Philippines. J SE Asian Earth Sci 6:221–238
Ayliffe MA, Timmis JN (1992) Tobacco nuclear DNA contains long tracts of homology to chloroplast DNA. Theor Appl Genet 85:229–238
Ayliffe MA, Scott NS, Timmis JN (1998) Analysis of plastid DNA-like sequences within the nuclear genomes of higher plants. Mol Biol Evol 15:738–745
Carlsward BS, Whitten WM, Williams NH, Bytebier B (2006) Molecular phylogenetics of Vandeae (Orchidaceae) and the evolution of leaflessness. Amer J Bot 93:770–786
Chen WH, Fu YM, Hsieh RM, Tsai WT, Chou MS, Wu CC, Lin YS (1995) Application of DNA amplification fingerprinting in the breeding of Phalaenopsis orchid. In: Terzi M, Cella R, Falavigna A (eds) Current issues in plant molecular and cellular biology. Kluwer Academic Publishers, Dordrecht, pp 341–346
Cheung WY, Scott NS (1989) A contiguous sequence in spinach nuclear DNA is homologous to three separated sequences in chloroplast DNA. Theor Appl Genet 77:625–633
Christenson EA (1986) Nomenclatural changes in the Orchidaceae subtribe Sarcanthinae. Selbyana 9:167–170
Christenson EA (2001) Phalaenopsis. Timber Press, Portland, p 330
Christenson EA, Whitten MW (1995) Phalaenopsis bellina (Rchb.f.) Christenson, a segregate from P. violacea Witte (Orchidaceae: Aeridinae). Brittonia 47:57–60
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Dressler RL (1993) Phylogeny and classification of the orchid family. Dioscorides Press, Portland, p 314
Ellis J (1982) Promiscuous DNA—chloroplast genes inside plant mitochondria. Nature 299:678–679
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fitch WM (1971) Towards defining the course of evolution: minimum change for a specific tree topology. Syst Zool 20:406–416
Fowlie JA (1983) A new Phalaenopsis species of the section Zebrinae from central Sumatra, Phalaenopsis inscriptiosinensis Fowl. Orchid Dig 47:11–12
Fowlie JA (1993) A new species of Phalaenopsis from Flores Island. Indonesia: Phalaenopsis floresensis Fowl., sp. nov. Orchid Dig 57:35–36
Garay LA, Hamer F, Siegerist ES (1995) Inquilina orchidacea: Orchidaceae plaerumque Levyanae. Lindleyana 10:174–182
Goh MWK, Kumar PP, Lim SH, Tan HTW (2005) Random amplified polymorphic DNA analysis of the moth orchids, Phalaenopsis (Epidendroideae: Orchidaceae). Euphytica 141:11–22
Gruss O, Rollke L (1991) Eine weitere Phalaenopsis von den Philippinen-P. bastianii Gruss and Rollke. Die Orchidee 42:76–79
Hall R (1996) Reconstructing Cenozoic SE Asia. In: Hall R, Blundell DJ (eds) Tectonic evoultion of SE Asia. Geological Society of London Special Publication. No. 106, pp 153–184
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hanebuth T, Stattegger K, Grootes PM (2000) Rapid flooding of the Sunda Shelf: a late-glacial sea-level record. Science 288:1033–1035
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192
Holttum RE (1959) Evolutionary trends in the Sarcanthiine orchids. Am Orchid Soc Bull 5:399–423
Huang CY, Grunheit N, Ahmadinejad N, Timmis JN, Martin W (2005) Mutational decay and age of chloroplast and mitochondrial genomes transferred recently to angiosperm nuclear chromosomes. Plant Physiol 138:1723–1733
Kao YH (2001) Phylogeny of Phalaenopsis species based on 5S rDNA intergenic sequences. Master’s thesis, Institute of Botany National Taiwan University, Taipei, Taiwan (in Chinese with English abstract)
Kao YY, Chang SB, Lin TY, Hsieh CH, Chen YH, Chen WH, Chen CC (2001) Differential accumulation of heterochromatin as a cause for karyotype variation in Phalaenopsis orchids. Ann Bot 87:387–395
Karig DE, Sarewitz DR, Iiaeck GD (1986) Role of strike-slip faulting in the evolution of allochthonous terranes in the Philippines. Geology 14:852–855
Kocyan A, de Vogel EF, Conti E, Gravendeel B (2008) Molecular phylogeny of Aerides (Orchidaceae) based on one nuclear and two plastid markers: a step forward in understanding the evolution of the Aeridinae. Mol Phylogenet Evol 48:422–443
Lin S, Lee HC, Chen WH, Chen CC, Kao YY, Fu YM, Chen YH, Lin TY (2001) Nuclear DNA contents of Phalaenopsis species and Doritis pulcherrima. J Am Soc Hort Sci 126:195–199
Liu F (1988) A new species of Phalaenopsis from Yunnan. Acta Bot Yunnan 10:119–120
Liu Y, Zhang Q, Hu Y, Sodmergen (2004) Heterogeneous pollen in Chlorophytum comosum, a species with a unique mode of plastid inheritance intermediate between the maternal and biparental modes. Plant Physiol 135:193–200
Matsushima R, Hu Y, Toyoda K, Sodmergen, Sakamoto W (2008) The model plant Medicago truncatula exhibits biparental plastid inheritance. Plant Cell Physiol 49:81–91
Padolina J, Linder CR, Simpson BB (2005) A phylogeny of Phalaenopsis using multiple chloroplast markers. Selbyana 26:23–27
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Quebral RD, Pubellier M, Rangin C (1994) The Mindanao: a transition from collision to strike-slip environment. Tectonics 15:713–726
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rzhetsky A, Nei M (1992) Statistical properties of the ordinary least-squares, generalized least-squares, and minimum-evolution methods of phylogenetic inference. J Mol Evol 35:367–375
Sathiamurthy E, Voris HK (2006) Maps of Holocene sea transgression and submerged lakes on the Sunda Shelf. Nat Hist J Chulalongkorn Univ Supplementary 2:1–43
Second G, Dally A, Zhang SH (1989) Occasional biparent inheritance of chloroplast DNA in rice. Rice Genet Newsl 6:150–152
Seidenfaden G (1988a) Kingidium. Opera Bot 95:182–189
Seidenfaden G (1988b) Doritis. Opera Bot 95:31–34
Shim PS (1982) A new generic classification in the Phalaenopsis complex (Orchidaceae). Malayan Nat J 36:1–28
Shim PS, Fowlie JA (1983) A new species of Phalaenopsis from Sulawesi (Celebes) formerly confused with Phalaenopsis psilantha Schltr., Phalaenopsis venosa Shim and Fowl. Orchid Dig 47:124–128
Shindo K, Kamemoto H (1963) Karyotype analysis of some species of Phalaenopsis. Cytologia 28:390–398
Siegerist ES (1989) Kingidium deliciosum. Am Orchid Soc Bull 56:228–231
Stephan JF, Blanchet R, Rangin C, Pelletir B, Letouzey J, Muller C (1986) Geodynamic evolution of the Taiwan-Luzon-Mindoro belt since the Late Eocene. Tectonophysics 125:245–268
Sweet HR (1980) The genus Phalaenopsis. The Orchid Digest, Pomona, p 128
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tharp AG, Fowlie JA, Tang CZ (1987) A recently described Phalaenopsis species from the Philippines: Phalaenopsis philippinensis Golamco ex Fowl. and Tang. Orchid Dig 51:87–92
Timmis JN, Scott NS (1983) Sequence homology between spinach nuclear and chloroplast genomes. Nature 305:65–67
Topik H, Yukawa T, Ito M (2005) Molecular phylogenetics of subtribe Aeridinae (Orchidaceae): insights from plastid matK and nuclear ribosomal ITS sequences. J Plant Res 118:271–284
Tsai CC, Huang SC, Huang PL, Chou CH (2003) Phylogeny of the genus Phalaenopsis (Orchidaceae) with emphasis on the subgenus Phalaenopsis based on the sequences of the internal transcribed spacers 1 and 2 of rDNA. J Hort Sci Biotech 78:879–887
Tsai CC, Huang SC, Chou CH (2006) Molecular phylogeny of Phalaenopsis Blume (Orchidaceae) based on the internal transcribed spacer of the nuclear ribosomal DNA. Plant Syst Evol 256:1–16
van Oosterzee P (1997) Where worlds collide: the Wallace line. Cornell University Press, Ithaca
Wolfe KH, Li WH, Sharp PM (1987) Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci USA 84:9054–9058
Yuan Q, Hill J, Hsiao J, Moffat K, Ouyang S, Cheng Z, Jiang J, Buell CR (2002) Genome sequencing of a 239-kb region of rice chromosome 10L reveals a high frequency of gene duplication and a large chloroplast DNA insertion. Mol Genet Genomics 267:713–720
Yukawa T (1996) Phalaenopsis chibae (Orchidaceae)–a new species from Vietnam. Ann Tsukuba Bot Gard 15:19–22
Yukawa T, Kita K, Handa T, Topik H (2005) Molecular phylogenetics of Phalaenopsis (Orchidaceae) and allied genera: re-evaluation of generic concepts. Acta Phytotax Geobot 56:141–161
Acknowledgments
The authors thank Dr James Beck and Dr Yi-Shan Chao for their valuable comments and helpful discussion, which improved the manuscript. This research was supported by funding from the National Science Council, Executive Yuan, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. C. Tsai and Y. C. Chiang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tsai, C.C., Chiang, Y.C., Huang, S.C. et al. Molecular phylogeny of Phalaenopsis Blume (Orchidaceae) on the basis of plastid and nuclear DNA. Plant Syst Evol 288, 77–98 (2010). https://doi.org/10.1007/s00606-010-0314-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-010-0314-1