Abstract
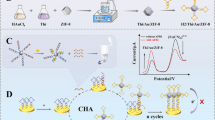
The influence of Au@Pt nanoparticles’ composition, morphology, and peroxidase-mimicking activity on the limit of detection (LOD) of lateral flow immunoassay (LFIA) has been investigated. Fourteen types of nanoparticles were synthesized by varying the concentration of Pt4+ (20–2000 μM), using gold nanoparticles (GNP, diameter 20.0 ± 2.6 nm) as the seeds and ascorbic acid as a reducing agent. Au@Pt nanoparticles and GNPs were conjugated with antibodies specific to the target analyte, a widespread and dangerous phytopathogenic bacteria species (Clavibacter michiganensis). We found that the 100-fold growth of the Pt4+ concentration was accompanied by an increase of the Au@Pt nanoparticle diameter (24–55 nm) and surface area with the formation of urchin-shaped morphology. These changes led to a 70-fold increase in peroxidase-mimicking activity in the solution (specific activity 0.06–4.4 U mg−1) and a 30-fold decrease in LOD using the catalytic activity of Au@Pt. The Au@Pt nanoparticles synthesized at 1000–2000 μM of Pt4+ demonstrated statistically indistinguishable catalytic activity. The highest sensitivity of LFIA was reached for Au@Pt nanoparticles synthesized at Pt4+ concentration equal to 1000 μM. Au@Pt nanoparticles saved most of their peroxidase-mimicking activity, whereas endogenous plant peroxidases were completely inhibited by sodium azide. The LOD of LFIA with Au@Pt nanoparticles synthesized at 1200 μM of Pt4+ was 300 colony-forming units (CFU) per mL of buffer and 500 CFU per mL of potato tuber extract, which provides 330- and 200-fold improvement compared to the conventional LFIA with GNPs. The assay consists of three rapid 5-min stages, namely, extraction, lateral flow, and color enhancement (oxidation of diaminobenzidine by Au@Pt nanoparticles). LFIA with the urchin Au@Pt nanoparticles allows the detection of latent bacterial infections rapidly without equipment or special skills.

Graphical abstract




Similar content being viewed by others
References
Zhu G, Yin X, Jin D et al (2019) Paper-based immunosensors: current trends in the types and applied detection techniques. TrAC Trends Anal Chem 111:100–117. https://doi.org/10.1016/j.trac.2018.09.027
Bishop JD, Hsieh HV, Gasperino DJ, Weigl BH (2019) Sensitivity enhancement in lateral flow assays: a systems perspective. Lab Chip 19:2486–2499. https://doi.org/10.1039/c9lc00104b
Yang H, Xu W, Zhou Y (2019) Signal amplification in immunoassays by using noble metal nanoparticles: a review. Microchim Acta 186:859. https://doi.org/10.1007/s00604-019-3904-9
Tang RH, Liu LN, Zhang SF, He XC, Li XJ, Xu F, Ni YH, Li F (2019) A review on advances in methods for modification of paper supports for use in point-of-care testing. Microchim Acta 186:357–325. https://doi.org/10.1007/s00604-019-3626-z
Zhou Y, Ding L, Wu Y et al (2019) Emerging strategies to develop sensitive AuNP-based ICTS nanosensors. TrAC Trends Anal Chem 112:147–160. https://doi.org/10.1016/j.trac.2019.01.006
Gao Z, Xu M, Lu M, Chen G, Tang D (2015) Urchin-like (gold core)@(platinum shell) nanohybrids: a highly efficient peroxidase-mimetic system for in situ amplified colorimetric immunoassay. Biosens Bioelectron 70:194–201. https://doi.org/10.1016/j.bios.2015.03.039
Loynachan CN, Thomas MR, Gray ER, Richards DA, Kim J, Miller BS, Brookes JC, Agarwal S, Chudasama V, McKendry R, Stevens MM (2018) Platinum nanocatalyst amplification: redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 12:279–288. https://doi.org/10.1021/acsnano.7b06229
Jiang T, Song Y, Wei T, Li H, du D, Zhu MJ, Lin Y (2016) Sensitive detection of Escherichia coli O157:H7 using Pt–Au bimetal nanoparticles with peroxidase-like amplification. Biosens Bioelectron 77:687–694. https://doi.org/10.1016/j.bios.2015.10.017
Zhang J, Yu Q, Qiu W, Li K, Qian L, Zhang X, Liu G (2019) Gold-platinum nanoflowers as a label and as an enzyme mimic for use in highly sensitive lateral flow immunoassays: application to detection of rabbit IgG. Microchim Acta 186:357. https://doi.org/10.1007/s00604-019-3464-z
Gao Z, Ye H, Tang D, Tao J, Habibi S, Minerick A, Tang D, Xia X (2017) Platinum-decorated gold nanoparticles with dual functionalities for ultrasensitive colorimetric in vitro diagnostics. Nano Lett 17:5572–5579. https://doi.org/10.1021/acs.nanolett.7b02385
Lu Y, Yu J, Ye W et al (2016) Spectrophotometric determination of mercury(II) ions based on their stimulation effect on the peroxidase-like activity of molybdenum disulfide nanosheets. Microchim Acta 183:2481–2489. https://doi.org/10.1007/s00604-016-1886-4
Panferov VG, Samokhvalov AV, Safenkova IV, Zherdev AV, Dzantiev BB (2018) Study of growth of bare and protein-modified gold nanoparticles in the presence of hydroxylamine and tetrachloroaurate. Nanotechnol Russ 13:614–622. https://doi.org/10.1134/S1995078018060095
Bragard C, Dehnen-Schmutz K, Di Serio F et al (2019) Pest categorisation of Clavibacter sepedonicus. EFSA J 17:4. https://doi.org/10.2903/j.efsa.2019.5670
A2 List of pests recommended for regulation as quarantine pests. Version 2019–09. European and Mediterranean Plant Protection Organization
Perminow JIS, Akselsen ILW, Borowski E, Ruden Ø, Grønås W (2012) Potato ring rot in Norway: occurrence and control. Potato Res 55:241–247. https://doi.org/10.1007/s11540-012-9219-4
Franc GD (1999) Persistence and latency of Clavibacter michiganensis subsp. sepedonicus in field-grown seed potatoes. Plant Dis 83:247–250. https://doi.org/10.1094/PDIS.1999.83.3.247
Khater M, de la Escosura-Muñiz A, Merkoçi A (2017) Biosensors for plant pathogen detection. Biosens Bioelectron 93:72–86. https://doi.org/10.1016/j.bios.2016.09.091
Safenkova IV, Zaitsev IA, Pankratova GK, Varitsev YA, Zherdev AV, Dzantiev BB (2014) Lateral flow immunoassay for rapid detection of potato ring rot caused by Clavibacter michiganensis subsp. sepedonicus. Appl Biochem Microbiol 50:675–682. https://doi.org/10.1134/S0003683814120011
Priou S, Gutarra L, Aley P (1999) Highly sensitive detection of Ralstonia solanacearum in latently infected potato tubers by post-enrichment enzyme-linked immunosorbent assay on nitrocellulose membrane. EPPO Bull 29:117–125. https://doi.org/10.1111/j.1365-2338.1999.tb00805.x
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22. https://doi.org/10.1038/physci241020a0
Jiang B, Duan D, Gao L, Zhou M, Fan K, Tang Y, Xi J, Bi Y, Tong Z, Gao GF, Xie N, Tang A, Nie G, Liang M, Yan X (2018) Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat Protoc 13:1506–1520. https://doi.org/10.1038/s41596-018-0001-1
Bigall NC, Härtling T, Klose M, Simon P, Eng LM, Eychmüller A (2008) Monodisperse platinum nanospheres with adjustable diameters from 10 to 100 nm: synthesis and distinct optical properties. Nano Lett 8:4588–4592. https://doi.org/10.1021/nl802901t
Safenkova I, Zherdev A, Dzantiev B (2012) Factors influencing the detection limit of the lateral-flow sandwich immunoassay: a case study with potato virus X. Anal Bioanal Chem 403:1595–1605. https://doi.org/10.1007/s00216-012-5985-8
Cui X, Huang Y, Wang J et al (2015) A remarkable sensitivity enhancement in a gold nanoparticle-based lateral flow immunoassay for the detection of Escherichia coli O157:H7. RSC Adv 5:45092–45097. https://doi.org/10.1039/C5RA06237C
Serebrennikova K, Samsonova J, Osipov A (2018) Hierarchical nanogold labels to improve the sensitivity of lateral flow immunoassay. Nano-Micro Lett 10:1–8. https://doi.org/10.1007/s40820-017-0180-2
Wu J, Wang X, Wang Q et al (2019) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev 48:1004–1076. https://doi.org/10.1039/C8CS00457A
Zhao C, Xiong C, Liu X, Qiao M, Li Z, Yuan T, Wang J, Qu Y, Wang X, Zhou F, Xu Q, Wang S, Chen M, Wang W, Li Y, Yao T, Wu Y, Li Y (2019) Unraveling the enzyme-like activity of heterogeneous single atom catalyst. Chem Commun 55:2285–2288. https://doi.org/10.1039/C9CC00199A
Niu X, Shi Q, Zhu W, Liu D, Tian H, Fu S, Cheng N, Li S, Smith JN, du D, Lin Y (2019) Unprecedented peroxidase-mimicking activity of single-atom nanozyme with atomically dispersed Fe–Nx moieties hosted by MOF derived porous carbon. Biosens Bioelectron 142:111495. https://doi.org/10.1016/j.bios.2019.111495
Duan D, Fan K, Zhang D, Tan S, Liang M, Liu Y, Zhang J, Zhang P, Liu W, Qiu X, Kobinger GP, Gao GF, Yan X (2015) Nanozyme-strip for rapid local diagnosis of Ebola. Biosens Bioelectron 74:134–141. https://doi.org/10.1016/j.bios.2015.05.025
Safenkova IV, Slutskaya ES, Panferov VG, Zherdev AV, Dzantiev BB (2016) Complex analysis of concentrated antibody-gold nanoparticle conjugates’ mixtures using asymmetric flow field-flow fractionation. J Chromatogr A 1477:56–63. https://doi.org/10.1016/j.chroma.2016.11.040
Acknowledgments
The authors are grateful to Yu.A. Varitsev (A.G. Lorch All-Russian Potato Research Institute, Korenevo, Moscow region, Russia) for providing the infected and healthy plants, N.V. Drenova (All-Russian Plant Quarantine Centre, Bykovo-2, Moscow region, Russia) for providing bacterial strains, and E.S. Slutskaya (Federal Research Centre “Fundamentals of Biotechnology” of the Russian Academy of Sciences, Moscow, Russia) for the ICP MS measurements.
Funding
This study was financially supported by the Russian Science Foundation (grant no. 16-16-04108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 3220 kb)
Rights and permissions
About this article
Cite this article
Panferov, V.G., Safenkova, I.V., Zherdev, A.V. et al. Urchin peroxidase-mimicking Au@Pt nanoparticles as a label in lateral flow immunoassay: impact of nanoparticle composition on detection limit of Clavibacter michiganensis. Microchim Acta 187, 268 (2020). https://doi.org/10.1007/s00604-020-04253-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04253-3