Abstract
This work describes an aptasensor for the foodborne pathogen Shigella dysenteriae (S. dysenteriae). A glassy carbon electrode (GCE) was modified with gold nanoparticles (AuNPs) by electrodeposition. Then, thiolated aptamer for S. dysenteriae detection was self-assembled on the surface of the modified GCE, and any free residual AuNPs were blocked with 6-mercapto-1-hexanol. The size, morphology, and distribution of the AuNPs were characterized by field emission scanning electron microscopy. Detection of S. dysenteriae was performed measurement of the charge transfer resistance (Rct) before and after addition of S. dysenteriae using hexacyanoferrate as an electrochemical probe. The interaction between the aptamer and outer-membrane proteins of S. dysenteriae lead to an increase in the Rct of the sensor. The assay has a linear dynamic range that extends from 101 to 106 CFU.mL−1 and a limit of detection of 100 CFU.mL−1. It can differentiate between alive S. dysenteriae and other pathogens. Dead S. dysenteriae cells do not have any effect on selectivity. Unpasteurized and pasteurized skim milk and some water samples were spiked with S. dysenteriae and then successfully examined by this method. The results were validated by real-time PCR. The method is fast, low-cost, highly sensitive, and specific. Hence, it represents a valuable tool in food quality control.

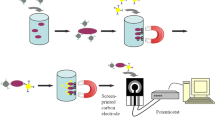
Schematic presentation of a label free impedimetric aptasensor for Shigella dysenteriae using a glassy carbon electrode modified with gold nanoparticles (AuNPs) and 6-mercapto-1-hexanol (MCH). The limit of detection of this aptasensor is as low as 1 CFU.mL−1 for target bacteria.



Similar content being viewed by others
References
The HC, Thanh DP, Holt KE, Thomson NR, Baker S (2016) The genomic signatures of Shigella evolution, adaptation and geographical spread. Nat Rev Microbiol 14:235–250. https://doi.org/10.1038/nrmicro.2016.10
Duan N, Ding X, Wu S, Xia Y, Ma X, Wang Z, Chen J (2013) In vitro selection of a DNA aptamer targeted against Shigella dysenteriae. J Microbiol Methods 94:170–174. https://doi.org/10.1016/j.mimet.2013.06.016
WHO (2017) Guidelines for Drinking-water Quality, fourth. Geneva
FDA (2018) Health Educators - Food Safety for Moms-to-Be: Medical Professionals - Foodborne Pathogens. Center for Food Safety and Applied Nutrition. https://www.fda.gov/food/resourcesforyou/healtheducators/ucm091681.htm. Accessed 19 october 2007
Bagheryan Z, Raoof J-B, Golabi M, Turner APF, Beni V (2016) Diazonium-based impedimetric aptasensor for the rapid label-free detection of Salmonella typhimurium in food sample. Biosens Bioelectron 80:566–573. https://doi.org/10.1016/j.bios.2016.02.024
Izadi Z, Sheikh-Zeinoddin M, Ensafi AA, Soleimanian-Zad S (2016) Fabrication of an electrochemical DNA-based biosensor for Bacillus cereus detection in milk and infant formula. Biosens Bioelectron 80:582–589. https://doi.org/10.1016/j.bios.2016.02.032
Zelada-Guillen GA, Riu J, Düzgün A, Rius FX (2009) Immediate detection of living bacteria at ultralow concentrations using a carbon nanotube based potentiometrie aptasensor. Angew Chemie - Int Ed 48:7334–7337. https://doi.org/10.1002/anie.200902090
Li L, Yuan Y, Chen Y, Zhang P, Bai Y, Bai L (2018) Aptamer based voltammetric biosensor for Mycobacterium tuberculosis antigen ESAT-6 using a nanohybrid material composed of reduced graphene oxide and a metal-organic framework. Microchim Acta 185:379. https://doi.org/10.1007/s00604-018-2884-5
Templier V, Roux A, Roupioz Y, Livache T (2016) Ligands for label-free detection of whole bacteria on biosensors: a review. TrAC Trends Anal Chem 79:71–79. https://doi.org/10.1016/j.trac.2015.10.015
Liang G, Man Y, Jin X, Pan L, Liu X (2016) Aptamer-based biosensor for label-free detection of ethanolamine by electrochemical impedance spectroscopy. Anal Chim Acta 936:222–228. https://doi.org/10.1016/j.aca.2016.06.056
Rezaei B, Jamei HR, Ensafi AA (2018) Lysozyme aptasensor based on a glassy carbon electrode modified with a nanocomposite consisting of multi-walled carbon nanotubes, poly(diallyl dimethyl ammonium chloride) and carbon quantum dots. Microchim Acta 185:180. https://doi.org/10.1007/s00604-017-2656-7
Jia F, Duan N, Wu S, Ma X, Xia Y, Wang Z, Wei X (2014) Impedimetric aptasensor for Staphylococcus aureus based on nanocomposite prepared from reduced graphene oxide and gold nanoparticles. Microchim Acta 181:967–974. https://doi.org/10.1007/s00604-014-1195-8
Kaur H, Shorie M, Sharma M, Ganguli AK, Sabherwal P (2017) Bridged rebar graphene functionalized aptasensor for pathogenic E. coli O78:K80:H11 detection. Biosens Bioelectron 98:486–493. https://doi.org/10.1016/j.bios.2017.07.004
Roushani M, Shahdost-Fard F (2015) Fabrication of an ultrasensitive ibuprofen nanoaptasensor based on covalent attachment of aptamer to electrochemically deposited gold-nanoparticles on glassy carbon electrode. Talanta 144:510–516. https://doi.org/10.1016/j.talanta.2015.06.052
Ma Y, Liu J, Li H (2017) Diamond-based electrochemical aptasensor realizing a femtomolar detection limit of bisphenol a. Biosens Bioelectron 92:21–25. https://doi.org/10.1016/j.bios.2017.01.041
Heydari-Bafrooei E, Amini M, Saeednia S (2017) Electrochemical detection of DNA damage induced by bleomycin in the presence of metal ions. J Electroanal Chem 803:104–110. https://doi.org/10.1016/j.jelechem.2017.09.031
Labib M, Zamay AS, Kolovskaya OS, Reshetneva IT, Zamay GS, Kibbee RJ, Sattar SA, Zamay TN, Berezovski MV (2012) Aptamer-based viability impedimetric sensor for bacteria. Anal Chem 84:8966–8969. https://doi.org/10.1021/ac302902s
Liu PY, Chin LK, Ser W, Ayi TC, Yap PH, Bourouina T, Leprince-Wang Y (2014) An optofluidic imaging system to measure the biophysical signature of single waterborne bacteria. Lab Chip 14:4237–4243. https://doi.org/10.1039/C4LC00783B
Sheikhzadeh E, Chamsaz M, Turner APF, Jager EWH, Beni V (2016) Label-free impedimetric biosensor for Salmonella Typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens Bioelectron 80:194–200. https://doi.org/10.1016/j.bios.2016.01.057
Jia F, Duan N, Wu S, Dai R, Wang Z, Li X (2016) Impedimetric Salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim Acta 183:337–344. https://doi.org/10.1007/s00604-015-1649-7
Luo J, Wang J, Mathew AS, Yau ST (2016) Ultrasensitive detection of Shigella species in blood and stool. Anal Chem 88:2010–2014. https://doi.org/10.1021/acs.analchem.5b04242
Ma K, Deng Y, Bai Y, Xu D, Chen E, Wu H, Li B, Gao L (2014) Rapid and simultaneous detection of Salmonella, Shigella, and Staphylococcus aureus in fresh pork using a multiplex real-time PCR assay based on immunomagnetic separation. Food Control 42:87–93. https://doi.org/10.1016/j.foodcont.2014.01.042
Tang X-J, Yang Z, Chen X-B, Tian W-F, Tu C-N, Wang H-B (2018) Verification and large scale clinical evaluation of a national standard protocol for Salmonella spp ./Shigella spp. screening using real-time PCR combined with guided culture. J Microbiol Methods 145:14–19. https://doi.org/10.1016/j.mimet.2017.12.007
Kumar S, Balakrishna K, Batra HV (2008) Enrichment-ELISA for detection of Salmonella typhi from food and water samples. Biomed Environ Sci 21:137–143. https://doi.org/10.1016/S0895-3988(08)60019-7
Febo TD, Schirone M, Visciano P, Portanti O, Armillotta G, Persiani T, Giannatale E, Tittarelli M, Luciani M (2018) Development of a capture ELISA for rapid detection of Salmonella enterica in food samples. Food Anal Methods 2018. https://doi.org/10.1007/s12161-018-1363-2
Shan S, Liu D, Guo Q, Wu S, Chen R, Luo K, Hu L, Xiong Y, Lai W (2016) Sensitive detection of Escherichia coli O157:H7 based on cascade signal amplification in ELISA. J Dairy Sci 99:7025–7032. https://doi.org/10.3168/jds.2016-11320
Li Y, Cao L, Zhang C, Chen Q, Lu F, Bie X, Lu Z (2013) Development and evaluation of a PCR-ELISA assay for the detection and quanti fi cation of Cronobacter spp. Int Dairy J 33:27–33. https://doi.org/10.1016/j.idairyj.2013.06.009
Ojha SC, Yean Yean C, Ismail A, Banga Singh KK (2013) A pentaplex PCR assay for the detection and differentiation of shigella species. Biomed Res Int 2013:412370. https://doi.org/10.1155/2013/412370
Zhong QP, Wang L, Wang B, Chen HY (2011) Loop-mediated isothermal amplification method for rapid detection of Shigella dysenteriae. Appl Mech Mater 140:369–373. https://doi.org/10.4028/www.scientific.net/AMM.140.369
Du M, Li J, Zhao R, Yang Y, Wang Y, Ma K, Cheng X, Wan Y, Wu X (2018) Effective pre-treatment technique based on immune-magnetic separation for rapid detection of trace levels of Salmonella in milk. Food Control 91:92–99. https://doi.org/10.1016/j.foodcont.2018.03.032
Acknowledgements
Authors wish to thank the Research Institute of Biotechnology and Bioengineering for supporting this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 209 kb)
Rights and permissions
About this article
Cite this article
Zarei, S.S., Soleimanian-Zad, S. & Ensafi, A.A. An impedimetric aptasensor for Shigella dysenteriae using a gold nanoparticle-modified glassy carbon electrode. Microchim Acta 185, 538 (2018). https://doi.org/10.1007/s00604-018-3075-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3075-0