Abstract
The 6-kDa early secretory antigenic target referred to as ESAT-6 is a virulence factor secreted by Mycobacterium tuberculosis (MTB). This work describes a voltammetric aptasensor for ultrasensitive detection of ESAT-6. Reduced graphene oxide doped with metal-organic framework (MOF-rGO) was deposited on a glassy carbon electrode (GCE). This increases the immobilization of electroactive Toluidine Blue (TB) and facilitates the electron transfer from TB to the modified GCE. Platinum/gold core/shell (Pt@Au) nanoparticles were used to assemble thiolated ESAT-6 binding aptamer (EBA) on a modified electrode and to further amplify the response to TB. The modified GCE, typically operated at −0.36 V (vs. SCE), has a linear response in 1.0 × 10−4 to 2.0 × 102 ng⋅mL-1 ESAT-6 concentration range, and the limit of detection (LOD) for ESAT-6 is as low as 3.3 × 10−5 ng⋅mL-1. It exhibits satisfactory specificity and reproducibility when analyzing spiked human serum.

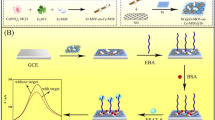
Schematic presentation of a voltammetric aptasensor for Mycobacterium tuberculosis antigen ESAT-6 using a glassy carbon electrode modified with reduced graphene oxide (rGO) and a metal-organic framework (MOF). The limit of detection for ESAT-6 is as low as 3.3 × 10−5 ng/mL.






Similar content being viewed by others
References
Zumla A, George A, Sharma V, Herbert R, BMo I, Oxley A, Oliver M (2015) The WHO 2014 global tuberculosis report--further to go. Lancet Glob Health 3(1):e10–e12
Golub JE, Bur S, Cronin WA, Gange S, Baruch N, Comstock GW, Chaisson RE (2006) Delayed tuberculosis diagnosis and tuberculosis transmission. Int J Tuberc Lung Dis 10(1):24–30
Elmasry S, Elkady I, Zaghloul MH, Albadrawey MK (2008) Rapid and simple detection of a mycobacterium circulating antigen in serum of pulmonary tuberculosis patients by using a monoclonal antibody and Fast-Dot-ELISA. Clin Biochem 41(3):145–151
Slogotskaya L, Bogorodskaya E, Sentchichina O, Nikitina G, Litvinov V, Seltsovsky P, Nikolenko N, Kudlay D (2016) Detection TB infection using a skin tests with tuberculin and allergen recombinant (CFP-10-ESAT-6) in children and adolescents in Moscow in 2015. Eur Respir J 48(suppl 60):OA3038
Wolf H, Mendez M, Gilman RH, Sheen P, Soto G, Velarde AK, Zimic M, Escombe AR, Montenegro S, Oberhelman RA (2008) Diagnosis of pediatric pulmonary tuberculosis by stool PCR. Am J Trop Med Hyg 79(6):893–898
Sarmiento OL, Weigle KA, Alexander J, Weber DJ, Miller WC (2003) Assessment by meta-analysis of PCR for diagnosis of smear-negative pulmonary tuberculosis. J Clin Microbiol 41(7):3233–3240
Zhang SL, Zhao JW, Sun ZQ, Yang EZ, Yan JH, Qi Z, Zhang GL, Zhang HM, Qi YM, Wang HH (2009) Development and evaluation of a novel multiple-antigen ELISA for serodiagnosis of tuberculosis. Tuberculosis 89(4):278–284
Doherty TM, Demissie A, Menzies D, Andersen P, Rook G, Zumla A (2005) Effect of sample handling on analysis of cytokine responses to Mycobacterium tuberculosis in clinical samples using ELISA, ELISPOT and quantitative PCR. J Immunol Methods 298(1–2):129–141
Goletti D, Petruccioli E, Joosten SA, Ottenhoff THM (2016) Tuberculosis biomarkers: from diagnosis to protection. Infect Dis Rep 8(2):24–32
Tang XL, Zhou YX, Wu SM, Pan Q, Xia B, Zhang XL (2014) CFP10 and ESAT6 aptamers as effective mycobacterial antigen diagnostic reagents. J Infect 69(6):569–580
Pollock JM, Andersen P (1997) The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J Infect Dis 175(5):1251–1254
Hill PC, Jacksonsillah D, Fox A, Franken KL, Lugos MD, Jeffries DJ, Donkor SA, Hammond AS, Adegbola RA, Ottenhoff TH (2005) ESAT-6/CFP-10 fusion protein and peptides for optimal diagnosis of mycobacterium tuberculosis infection by ex vivo enzyme-linked immunospot assay in the Gambia. J Clin Microbiol 43(5):2070–2074
Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR (2004) Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol 51(2):359–370
Diouani MF, Ouerghi O, Refai A, Belgacem K, Tlili C, Laouini D, Essafi M (2016) Detection of ESAT-6 by a label free miniature immuno-electrochemical biosensor as a diagnostic tool for tuberculosis. Mater Sci Eng C 74:465–470
Sepulveda D, Aroca MA, Varela A, Del PP, Osma JF (2017) Bioelectrochemical detection of Mycobacterium tuberculosis ESAT-6 in an antibody-based biomicrosystem. Sensors 17(10):2178
Cho EJ, Lee JW, Ellington AD (2009) Applications of Aptamers as sensors. Annu Rev Anal Chem 2(1):241–264
Famulok M, Mayer G (2011) Aptamer modules as sensors and detectors. Acc Chem Res 44(12):1349–1358
Bai L, Chai Y, Pu X, Yuan R (2014) A signal-on electrochemical aptasensor for ultrasensitive detection of endotoxin using three-way DNA junction-aided enzymatic recycling and graphene nanohybrid for amplification. Nanoscale 6(5):2902–2908
Wang G, Su X, Xu Q, Xu G, Lin J, Luo X (2017) Antifouling aptasensor for the detection of adenosine triphosphate in biological media based on mixed self-assembled aptamer and zwitterionic peptide. Biosens Bioelectron 101:129–134
Bai L, Yuan R, Chai Y, Zhuo Y, Yuan Y, Wang Y (2012) Simultaneous electrochemical detection of multiple analytes based on dual signal amplification of single-walled carbon nanotubes and multi-labeled graphene sheets. Biomaterials 33(4):1090–1096
Bai L, Chen Y, Bai Y, Chen Y, Zhou J, Huang A (2017) Fullerene-doped polyaniline as new redox nanoprobe and catalyst in electrochemical aptasensor for ultrasensitive detection of Mycobacterium tuberculosis MPT64 antigen in human serum. Biomaterials 133:11–19
Li WH, Ding K, Tian HR, Yao MS, Nath B, Deng WH, Wang Y, Xu G (2017) Conductive metal-organic framework nanowire array electrodes for high-performance solid-state supercapacitors. Adv Funct Mater 27(27):1702067
Sheberla D, Bachman JC, Elias JS, Sun CJ, Yang SH, Dincă M (2017) Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat Mater 16(2):220–224
Ke F, Wang L, Zhu J (2015) Multifunctional Au-Fe3O4@MOF core-shell nanocomposite catalysts with controllable reactivity and magnetic recyclability. Nanoscale 7(3):1201–1208
Tang Z, He J, Chen J, Niu Y, Zhao Y, Zhang Y, Yu C (2018) A sensitive sandwich-type immunosensor for the detection of galectin-3 based on N-GNRs-Fe-MOFs@AuNPs nanocomposites and a novel AuPt-methylene blue nanorod. Biosens Bioelectron 101:253–259
Guo Y, Sun X, Liu Y, Wang W, Qiu H, Gao J (2012) One pot preparation of reduced graphene oxide (RGO) or Au (Ag) nanoparticle-RGO hybrids using chitosan as a reducing and stabilizing agent and their use in methanol electrooxidation. Carbon 50(7):2513–2523
Wang Y, Hu S (2006) A novel nitric oxide biosensor based on electropolymerization poly(toluidine blue) film electrode and its application to nitric oxide released in liver homogenate. Biosens Bioelectron 22(1):10–17
Jia H, Chang G, Shu H, Xu M, Wang X, Zhang Z, Liu X, He H, Wang K, Zhu R (2017) Pt nanoparticles modified Au dendritic nanostructures: facile synthesis and enhanced electrocatalytic performance for methanol oxidation. Int J Hydrog Energy 42(34):22100–22107
Ataeeesfahani H, Wang L, Nemoto Y, Yamauchi Y (2010) Synthesis of bimetallic Au@Pt nanoparticles with Au Core and nanostructured Pt Shell toward highly active Electrocatalysts. Chem Mater 22(23):6310–6318
Thakur H, Kaur N, Sabherwal P, Sareen D, Prabhakar N (2017) Aptamer based voltammetric biosensor for the detection of Mycobacterium tuberculosis antigen MPT64. Microchim Acta 184(7):1–8
Thakur H, Kaur N, Sareen D, Prabhakar N (2017) Electrochemical determination of M. tuberculosis antigen based on Poly(3,4-ethylenedioxythiophene) and functionalized carbon nanotubes hybrid platform. Talanta 171:115–123
Ma Z, Qin L, Wang Y (2007) Screening and affinity analysis of aptamers to ESAT-6 protein from Mycobacterium tuberculosis. Chin J Clin 1:45–48
Ma Z, He F, Qin L, Hu Z, Jiang Q (2011) Selection of aptamers to CFP10-ESAT6 protein from Mycobacterium tuberculosis. J Mol Diagn Ther 3(6):377–380
Chen J, Yu C, Zhao Y, Niu Y, Zhang L, Yu Y, Wu J, He J (2016) A novel non-invasive detection method for the FGFR3 gene mutation in maternal plasma for a fetal achondroplasia diagnosis based on signal amplification by hemin-MOFs/PtNPs. Biosens Bioelectron 91:892–899
Wang H, Hu Q, Meng Y, Jin Z, Fang Z, Fu Q, Gao W, Xu L, Song Y, Lu F (2018) Efficient detection of hazardous catechol and hydroquinone with MOF-rGO modified carbon paste electrode. J Hazard Mater 353:151–157
Acknowledgements
This work is supported by National Natural Science Foundation of China (81601856), the Third Batch of Young Backbone Teachers Funding Program in Colleges and Universities of Chongqing City ([2016] No. 53), Funds for Young Science and Technology Talent Cultivation Plan of Chongqing City (cstc2014kjrc-qnrc00004) and Fundamental and Advanced Research Projects of Chongqing City (cstc2016jcyjA0275).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 3.23 mb)
Rights and permissions
About this article
Cite this article
Li, L., Yuan, Y., Chen, Y. et al. Aptamer based voltammetric biosensor for Mycobacterium tuberculosis antigen ESAT-6 using a nanohybrid material composed of reduced graphene oxide and a metal-organic framework. Microchim Acta 185, 379 (2018). https://doi.org/10.1007/s00604-018-2884-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2884-5