Abstract
A “turn on” time-resolved fluorometric aptasensor is described for the simultaneous detection of zearalenone (ZEN), trichothecenes A (T-2), and aflatoxin B1 (AFB1). Multicolor-emissive nanoparticles doped with lanthanide ions (Dy3+, Tb3+, Eu3+) were functionalized with respective aptamers and applied as a bioprobe, and tungsten disulfide (WS2) nanosheets are used as a quencher of time-resolved fluorescence. The assay exploits the quenching efficiency of WS2 and the interactions between WS2 and the respective DNA aptamers. The simultaneous recognition of the three mycotoxins can be performed in a single solution. In the absence of targets, WS2 is easily adsorbed by the mixed bioprobes via van der Waals forces between nucleobases and the WS2 basal plane. This brings the bioprobe and WS2 into close proximity and results in quenched fluorescence. In the presence of targets, the fluorescence of the bioprobes is restored because the analytes react with DNA probe and modify their molecular conformation to weaken the interaction between the DNAs and WS2. Under the optimum conditions and at an excitation wavelength of 273 nm, the time-resolved fluorescence intensities (peaking at 488, 544 and 618 nm and corresponding to emissions of Dy3+, Tb3+ and Eu3+) were used to quantify ZEN, T-2 and AFB1, respectively, with detection limits of 0.51, 0.33 and 0.40 pg mL−1 and a linear range from 0.001 to 100 ng mL−1. The three mycotoxins can be detected simultaneously without mutual interference. The assay was applied to the quantification of ZEN, T-2 and AFB1 in (spiked) maize samples. This homogeneous aptamer based assay can be performed within 1 h. Conceivably, it can become an alternative to other heterogeneous methods such as the respective enzyme-linked immunosorbent assays.

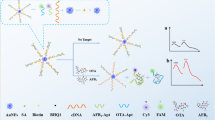
Schematic presentation of an aptasensor for simultaneous detection of zearalenone, trichothecenes A and aflatoxin B1 using aptamer modified time-resolved fluorescence nanoparticles as signalling probes and tungsten disulfide as the quencher. This assay shows lower detection limit and requires no washing steps.





Similar content being viewed by others
References
Grenier B, Oswald I (2011) Mycotoxin co-contamination of food and feed: meta-analysis of publications describing toxicological interactions. World Mycotoxin J 4(3):285–313. https://doi.org/10.3920/WMJ2011.1281
Chen Y, Meng X, Zhu Y, Shen M, Lu Y, Cheng J, Xu Y (2018) Rapid detection of four mycotoxins in corn using a microfluidics and microarray-based immunoassay system. Talanta 186:299–305. https://doi.org/10.1016/j.talanta.2018.04.064
Sun Y, Hu X, Zhang Y, Yang J, Wang F, Wang Y, Deng R, Zhang G (2014) Development of an immunochromatographic strip test for the rapid detection of zearalenone in corn. J Agric Food Chem 62(46):11116–11121. https://doi.org/10.1021/jf503092j
Gao X, Cao W, Chen M, Xiong H, Zhang X, Wang S (2014) A high sensitivity electrochemical sensor based on Fe3+-ion molecularly imprinted film for the detection of T-2 toxin. Electroanal 26(12):2739–2746. https://doi.org/10.1002/elan.201400237
Ma Y, Mao Y, Huang D, He Z, Yan J, Tian T, Shi Y, Song Y, Li X, Zhu Z (2016) Portable visual quantitative detection of aflatoxin B 1 using a target-responsive hydrogel and a distance-readout microfluidic chip. Lab Chip 16(16):3097–3104 https://pubs.rsc.org/en/content/articlehtml/2016/lc/c6lc00474a
Solfrizzo M, Gambacorta L, Lattanzio VM, Powers S, Visconti A (2011) Simultaneous LC–MS/MS determination of aflatoxin M 1, ochratoxin a, deoxynivalenol, de-epoxydeoxynivalenol, α and β-zearalenols and fumonisin B 1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal Bioanal Chem 401(9):2831. https://link.springer.com/article/10.1007/s00216-011-5354-z–2841
Tokuşoǧlu Ö, Ünal MK, Yemiş F (2005) Determination of the phytoalexin resveratrol (3, 5, 4 ‘-trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatography− mass spectrometry (GC-MS). J Agric Food Chem 53(12):5003–5009. https://doi.org/10.1021/jf050496+
Deng Q, Qiu M, Wang Y, Lv P, Wu C, Sun L, Ye R, Xu D, Liu Y, Gooneratne R (2017) A sensitive and validated immunomagnetic-bead based enzyme-linked immunosorbent assay for analyzing total T-2 (free and modified) toxins in shrimp tissues. Ecotoxicol Environ Saf 142:441–447 https://www.sciencedirect.com/science/article/pii/S0147651317302476
Zhang Z, Wang D, Li J, Zhang Q, Li P (2015) Monoclonal antibody–europium conjugate-based lateral flow time-resolved fluoroimmunoassay for quantitative determination of T-2 toxin in cereals and feed. Anal Methods 7(6):2822–2829 https://pubs.rsc.org/en/content/articlehtml/2015/ay/c5ay00100e
Wang D, Zhang Z, Li P, Zhang Q, Zhang W (2016) Time-resolved fluorescent immunochromatography of aflatoxin b1 in soybean sauce: a rapid and sensitive quantitative analysis. Sens 16(7):1094 https://www.mdpi.com/1424-8220/16/7/1094
Liu Y, Tu D, Zhu H, Chen X (2013) Lanthanide-doped luminescent nanoprobes: controlled synthesis, optical spectroscopy, and bioapplications. Chem Soc Rev 42(16):6924–6958 https://pubs.rsc.org/en/content/articlehtml/2013/cs/c3cs60060b
Li F, Zhang H, Wang Z, Newbigging AM, Reid MS, Li X-F, Le XC (2014) Aptamers facilitating amplified detection of biomolecules. Anal Chem 87(1):274–292. https://doi.org/10.1021/ac5037236
Zhou W, Huang P-JJ, Ding J, Liu J (2014) Aptamer-based biosensors for biomedical diagnostics. Anal 139(11):2627–2640 https://pubs.rsc.org/en/content/articlehtml/2014/an/c4an00132j
Huang Y, Zhang H, Chen X, Wang X, Duan N, Wu S, Xu B, Wang Z (2015) A multicolor time-resolved fluorescence aptasensor for the simultaneous detection of multiplex Staphylococcus aureus enterotoxins in the milk. Biosens Bioelectron 74:170–176. https://doi.org/10.1016/j.bios.2015.06.046
Huang Y, Chen X, Wu S, Duan N, Yu Y, Wang Z (2015) Homogeneous time-resolved fluorescence assay for the detection of ricin using an aptamer immobilized on europium-doped KGdF4 nanoparticles and graphene oxide as a quencher. Microchim Acta 182(5–6):1035–1043. https://doi.org/10.1007/s00604-014-1422-3
Wang X, Niazi S, Yukun H, Sun W, Wu S, Duan N, Hun X, Wang Z (2017) Homogeneous time-resolved FRET assay for the detection of Salmonella typhimurium using aptamer-modified NaYF4: Ce/Tb nanoparticles and a fluorescent DNA label. Microchim Acta 184(10):4021–4027. https://doi.org/10.1007/s00604-017-2399-5
Voiry D, Yamaguchi H, Li J, Silva R, Alves DC, Fujita T, Chen M, Asefa T, Shenoy VB, Eda G (2013) Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat Mater 12(9):850. https://www.nature.com/articles/nmat3700–855
Qin Y, Ma Y, Jin X, Zhang L, Ye G, Zhao S (2015) A sensitive fluorescence turn-on assay of bleomycin and nuclease using WS2 nanosheet as an effective sensing platform. Anal Chim Acta 866:84–89. https://doi.org/10.1016/j.aca.2015.01.049
Lin T, Zhong L, Song Z, Guo L, Wu H, Guo Q, Chen Y, Fu F, Chen G (2014) Visual detection of blood glucose based on peroxidase-like activity of WS2 nanosheets. Biosens Bioelectron 62:302–307. https://doi.org/10.1016/j.bios.2014.07.001
Ge J, Du Y-H, Chen J-J, Zhang L, Bai D-M, Ji D-Y, Hu Y-L, Li Z-H (2017) Highly sensitive fluorescence detection of mercury (II) ions based on WS2 nanosheets and T7 exonuclease assisted cyclic enzymatic amplification. Sensors Actuators B Chem 249:189–194. https://doi.org/10.1016/j.snb.2017.04.094
Zuo X, Zhang H, Zhu Q, Wang W, Feng J, Chen X (2016) A dual-color fluorescent biosensing platform based on WS2 nanosheet for detection of Hg2+ and Ag+. Biosens Bioelectron 85:464–470. https://doi.org/10.1016/j.bios.2016.05.044
Xi Q, Zhou D-M, Kan Y-Y, Ge J, Wu Z-K, Yu R-Q, Jiang J-H (2014) Highly sensitive and selective strategy for microRNA detection based on WS2 nanosheet mediated fluorescence quenching and duplex-specific nuclease signal amplification. Anal Chem 86(3):1361–1365. https://doi.org/10.1021/ac403944c
Chen X, Huang Y, Duan N, Wu S, Ma X, Xia Y, Zhu C, Jiang Y, Wang Z (2013) Selection and identification of ssDNA aptamers recognizing zearalenone. Anal Bioanal Chem 405(20):6573–6581. https://doi.org/10.1007/s00216-013-7085-9
Chen X, Huang Y, Duan N, Wu S, Xia Y, Ma X, Zhu C, Jiang Y, Wang Z (2014) Screening and identification of DNA aptamers against T-2 toxin assisted by graphene oxide. J Agric Food Chem 62(42):10368–10374. https://doi.org/10.1021/jf5032058
Ma X, Wang W, Chen X, Xia Y, Wu S, Duan N, Wang Z (2014) Selection, identification, and application of aflatoxin B1 aptamer. Eur Food Res Technol 238(6):919–925. https://doi.org/10.1007/s00217-014-2176-1
Ju Q, Tu D, Liu Y, Li R, Zhu H, Chen J, Chen Z, Huang M, Chen X (2011) Amine-functionalized lanthanide-doped KGdF4 nanocrystals as potential optical/magnetic multimodal bioprobes. J Am Chem Soc 134(2):1323–1330. https://doi.org/10.1021/ja2102604
Schäfer H, Ptacek P, Zerzouf O, Haase M (2008) Synthesis and optical properties of KYF4/Yb, Er nanocrystals, and their surface modification with undoped KYF4. Adv Funct Mater 18(19):2913–2918. https://doi.org/10.1002/adfm.200800368
Wu Z, Xu E, Chughtai MF, Jin Z, Irudayaraj J (2017) Highly sensitive fluorescence sensing of zearalenone using a novel aptasensor based on upconverting nanoparticles. Food Chem 230:673–680. https://doi.org/10.1016/j.foodchem.2017.03.100
Niazi S, Wang X, Pasha I, Khan IM, Zhao S, Shoaib M, Wu S, Wang Z (2018) A novel bioassay based on aptamer-functionalized magnetic nanoparticle for the detection of zearalenone using time resolved-fluorescence NaYF4: Ce/Tb nanoparticles as signal probe. Talanta 186:97–103. https://doi.org/10.1016/j.talanta.2018.04.013
Khan IM, Zhao S, Niazi S, Mohsin A, Shoaib M, Duan N, Wu S, Wang Z (2018) Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent detection of T-2 toxin. Sensors Actuators B Chem 277:328–335. https://doi.org/10.1016/j.snb.2018.09.021
Wang D, Zhang Z, Li P, Zhang Q, Ding X, Zhang W (2015) Europium nanospheres-based time-resolved fluorescence for rapid and ultrasensitive determination of total aflatoxin in feed. J Agric Food Chem 63(47):10313–10318. https://doi.org/10.1021/acs.jafc.5b03746
Zhang Z, Tang X, Wang D, Zhang Q, Li P, Ding X (2015) Rapid on-site sensing aflatoxin B1 in food and feed via a chromatographic time-resolved fluoroimmunoassay. PLoS One 10(4):e0123266. https://doi.org/10.1371/journal.pone.0123266
Sun L, Wu L, Zhao Q (2017) Aptamer based surface plasmon resonance sensor for aflatoxin B1. Microchim Acta 184(8):2605–2610. https://doi.org/10.1007/s00604-017-2265-5
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182(3–4):571–578. https://doi.org/10.1007/s00604-014-1360-0
Tang X, Li P, Zhang Q, Zhang Z, Zhang W, Jiang J (2017) Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and zearalenone in maize and its products. Anal Chem 89(21):11520–11528. https://doi.org/10.1021/acs.analchem.7b02794
Acknowledgements
This work was partly funded by the National Natural Science Foundation of China (31871881), Zhangjiagang Science and Technology Support plan (Social development) (ZKS 1803), Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF) (CX (18)2025), S&T Support Program of Jiangsu Province (BE2017623), the National First-class Discipline Program of Food Science and Technology (JUFSTR20180303), JUSRP51714B and the Distinguished Professor Program of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3.72 mb)
Rights and permissions
About this article
Cite this article
Niazi, S., Khan, I.M., Yu, Y. et al. A “turnon” aptasensor for simultaneous and time-resolved fluorometric determination of zearalenone, trichothecenes A and aflatoxin B1 using WS2 as a quencher. Microchim Acta 186, 575 (2019). https://doi.org/10.1007/s00604-019-3570-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3570-y