Abstract
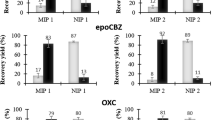
We have developed a solvent-free and sensitive method for the identification and quantification of methamphetamine (MAMP), amphetamine (AMP) and ecstasy (MDMA) in human urine. It is based on the use of an inside-needle adsorption trap (INAT) and a molecularly imprinted polymer (MIP). The MAMP-MIP layer was coated on the internal surface of a hollow stainless steel needle, which was oxidized and silylated. It was used as the extraction needle. A model solution containing the drugs was slowly passed through the extraction needle. After adsorption of the analytes, the needle was directly transferred to the injector of a gas chromatograph, where the analytes were thermally desorbed, separated by GC, and detected with a flame ionization detector. The method does not require an extraction solvent, is fast and simple. The linear range of the calibration graphs are rather wide, and the limit of detection and the limit of quantification (LOQ) for MAMP are 12 and 40 ng mL−1, respectively. The relative standard deviations (RSD%) for six repeated experiments (at 500 ng mL−1 of MAMP) is 4.9 %. The relative recoveries obtained for MAMP in spiked human urine samples are in the range of 81–93 %.

Typical chromatograms corresponding to the extraction of MAMP, AMP and MDMA in the optimum condition from human urine sample. Lower chromatogram (I) belong to non-spiked samples after extraction using MAMP-MIP coated needle and the other chromatograms, (II) and (III), are related to spiked samples with MAMP, AMP and MDMA (each 0.5 μg mL−1) and extraction using the NIP-coated needle and MAMP-MIP coated needle, respectively.




Similar content being viewed by others
References
Khajeamiri AR, Faizi M, Sohani F, Baheri T, Kobarfard F (2012) Determination of impurities in illicit methamphetamine samples seized in Iran. Forensic Sci Int 217:204–206
International Narcotics Control Board, Report 2009, United Nations, New York, 2010
Cook JD, Schanberg SM (1970) The effects of methamphetamine on behavior and on the uptake, release and metabolism of norepinephrine. Biochem Pharmacol 19:1162–1179
Sato M (1986) Acute exacerbation of methamphetamine psychosis and lasting dopaminergic. Psychopharmcol Bull 22:751–756
Kalant H (2001) The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. Can Med Assoc J 165:917–928
Huestis MA, Cho RE (2002) Drug abuse’s smallest victims: in utero drug exposure. Forensic Sci Int 128:20–30
Kumazawa T, Sato K, Seno H, Suzuki O (1993) Rapid extraction of methamphetamine and amphetamine in body fluids with bond elut SCX cartridges before capillary gas chromatography. Jpn J Legal Med 47:129–133
Myung SW, Min HK, Kim S, Kim M, Cho JB, Kim TJ (1998) Determination of amphetamine, methamphetamine and dimethamphetamine in human urine by solid-phase microextraction (SPME)-gas chromatography/mass spectrometry. J Chromatogr B 716:359–365
Raikos N, Christopoulou K, Theodoridis G, Tsoukali H, Psaroulis D (2003) Determination of amphetamines in human urine by headspace solid-phase microextraction and gas chromatography. J Chromatogr B 789:59–63
Hendrickson HP, Milesi-Halle A, Laurenzana EM, Owens SM (2004) Development of a liquid chromatography–tandem mass spectrometric method for the determination of methamphetamine and amphetamine using small volumes of rat serum. J Chromatogr B 806:81–87
Ming-Ren F, Ti-Yu W, Tzuen-Yeuan L (2006) Determination of amphetamine and methamphetamine in urine by solid phase extraction and ion-pair liquid chromatography–electrospray–tandem mass spectrometry. Talanta 68:987–991
Sato M, Mitsui T (1997) Rapid and simple determination of methamphetamine and amphetamine in blood by simultaneous extraction-derivatization. J Pharm Biomed Anal 16:139–145
Yamada H, Yamahara A, Yasuda S, Abe M, Oguri K, Fukushima S, Ikeda-Wada S (2002) Dansyl chloride derivatization of methamphetamine: a method with advantages for screening and analysis of methamphetamine in urine. J Anal Toxicol 266:17–22
Ramseier A, Caslavska J, Thormann W (1998) Screening for urinary amphetamine and analogs by capillary electrophoretic immunoassays and confirmation by capillary electrophoresis with on-column multiwave-length absorbance detection. Electrophoresis 19:2956–2966
Ramseier A, Siethoff C, Caslavska J, Thormann W (2000) Confirmation testing of amphetamines and designer drugs in human urine by capillary electrophoresis-ion trap mass spectrometry. Electrophoresis 21:380–387
Bagheri H, Mir A, Babanezhad E (2005) An electropolymerized aniline-based fiber coating for solid phase microextraction of phenols from water. Anal Chim Acta 532:89–95
Djozan D, Assadi Y (1999) Monitoring of polycyclic aromatic hydrocarbons in water using headspace solid-phase microextraction and capillary gas chromatography. Microchem J 63:276–284
Han D, Row KH (2012) Trends in liquid-phase microextraction, and its application to environmental and biological samples. Microchim Acta 176:1–22
Saraji M, Boroujeni MK (2011) Analysis of narcotic drugs in biological samples using hollow fiber liquid–phase microextraction and gas chromatography with nitrogen phosphorus detection. Microchim Acta 174:159–166
Djozan D, Baheri T, Pournaghi-Azar MH (2007) Development of electro solid-phase microextraction and application to methamphetamine analysis. Chromatographia 65:45–50
Djozan D, Farajzadeh MA, Sorouraddin SM, Baheri T (2011) Synthesis and application of high selective monolithic fibers based on molecularly imprinted polymer for SPME of trace methamphetamine. Chromatographia 73:975–983
Namera A, Yashiki M, Liu J, Okajima K, Hara K, Imamura T, Kojim T (2000) Simple and simultaneous analysis of fenfluramine, amphetamine and methamphetamine in whole blood by gas chromatography–mass spectrometry after headspace–solid phase microextraction and derivatization. Forensic Sci Int 109:215–223
Jochman MA, Kmiecik MP, Schmidt TC (2006) Solid-phase dynamic extraction for the enrichment of polar volatile organic compounds from water. J Chromatogr A 1115:208–216
Musshoff F, Madea B (2007) New trends in hair analysis and scientific demands on validation and technical notes. Forensic Sci Int 165:204–215
Eom IY, Niri VH, Pawliszyn J (2008) Development of a syringe pump assisted dynamic headspace sampling technique for needle trap device. J Chromatogr A 1196–1197:10–14
Eom IY, Tugulea AM, Pawliszyn J (2008) Development and application of needle trap devices. J Chromatogr A 1196–1197:3–9
Tamayo FG, Titirici MM, Esteban AM, Sellergren B (2005) Synthesis and evaluation of new propazine-imprinted polymer formats for use as stationary phases in liquid chromatography. Anal Chim Acta 542:38–46
Jiang X, Zhao C, Jiang N, Zhang H, Liu M (2008) Selective solid-phase extraction using molecular imprinted polymer for the analysis of diethylstilbestrol. Food Chem 108:1061–1067
Djozan D, Baheri T, Pournaghi Azar MH, Mahkam M (2007) Preparation of new fibers on the basis of codeine imprinted polymer. Mater Manuf Processes 22:758–763
Djozan D, Ebrahimi B (2008) Preparation of new solid phase micro extraction fiber on the basis of atrazine-molecular imprinted polymer: application for GC and GC/MS screening of triazine herbicides in water, rice and onion. Anal Chim Acta 616:152–159
Djozan D, Farajzadeh MA, Sorouraddin SM, Baheri T, Norouzi J (2012) Development of an inside needle extraction method based on molecularly imprinted polymer for solid-phase dynamic extraction and preconcentration of triazine herbicides followed by GC–FID determination. Chromatographia 75:139–148
Acknowledgments
The authors thank the Research Council of University of Tabriz for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 67 kb)
Rights and permissions
About this article
Cite this article
Djozan, D., Farajzadeh, M.A., Sorouraddin, S.M. et al. Determination of methamphetamine, amphetamine and ecstasy by inside-needle adsorption trap based on molecularly imprinted polymer followed by GC-FID determination. Microchim Acta 179, 209–217 (2012). https://doi.org/10.1007/s00604-012-0879-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0879-1