Abstract
We report on a method for trace analysis of the narcotic drugs alfentanil, fentanyl, and sufentanil in plasma and urine. Two–phase hollow fiber liquid–phase microextraction was combined with GC using nitrogen–phosphorus detection. Experimental parameters were optimized to give a viable analytical procedure whose limits of detection range from 8 to 15 ng L−1 (at an S/N of 3). The calibration curves are linear between 0.1 and 50 μg L−1, with squared correlation coefficients (r 2) between 0.9953 and 0.9979. Precision values range from 2.4% to 3.3% (intra–day RSD) and 3.2 to 6.3% (inter–day RSD). The relative recoveries varied from 27.8% to 84.6% (for spiked plasma) and 75 to 85.2% (for spiked urine). The method consumes little solvent, is simple, fast, inexpensive, and well suitable for the analysis of complicated matrices.

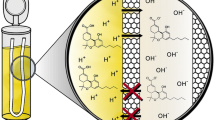
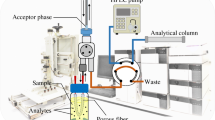
Schematic diagram of two phase hollow fiber liquid-phase microextraction (HF-LPME) combined with GC and nitrogen–phosphorus detection for trace analysis of the narcotic drugs alfentanil, fentanyl, and sufentanil in plasma and urine.




Similar content being viewed by others
References
Clotz MA, Nahata MC (1991) Clinical uses of fentanyl, sufentanil and alfentanil. Clin Pharm 10:581–593
Scholz J, Steinfath M, Schulz M (1996) Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil–an update. Clin Pharmacokinet 31:275–292
Valaer AK, Huber T, Andurkar SV, Clark CR, De Ruiter J (1997) Development of a gas chromatographic–mass spectrometric drug screening method for the N–dealkylated metabolites of fentanyl, sufentanil, and alfentanil. J Chromatogr Sci 35:461–466
Fryirsa B, Woodhouse A, Huang JL, Dawson M, Mather LE (1997) Determination of subnanogram concentrations of fentanyl in plasma by gas chromatography–mass spectrometry: comparison with standard radioimmunoassay. J Chromatogr B 688:79–85
Ruangyuttikarn W, Law MY, Rollins DE, Moody DE (1990) Detection of fentanyl and its analogs by enzyme–linked immunosorbent assay. J Anal Toxicol 14:160–164
Dotsikas Y, Loukas YL, Siafaka I (2002) Determination of umbilical cord and maternal plasma concentrations of fentanyl by using novel spectrophotometric and chemiluminescence enzyme immunoassays. Anal Chim Acta 459:177–185
Dotsikas Y, Loukas YL (2004) Employment of 4–(1–imidazolyl)phenol as a luminol signal enhancer in a competitive–type chemiluminescence immunoassay and its comparison with the conventional antigen–horseradish peroxidase conjugate–based assay. Anal Chim Acta 509:103–109
Raikos N, Theodoridis G, Alexiadou E, Gika H, Argiriadou H, Parlapani H, Tsoukali H (2009) Analysis of anesthetics and analgesics in human urine by headspace SPME and GC. J Sep Sci 32:1018–1026
Ebrahimzadeh H, Yamini Y, Gholizade A, Sedighi A, Kasraee S (2008) Determination of fentanyl in biological and water samples using single–drop liquid–liquid–liquid microextraction coupled with high–performance liquid chromatography. Anal Chim Acta 626:193–199
Wang C, Li E, Xu G, Wang H, Gong Y, Li P, Liu S, He Y (2009) Determination of fentanyl in human breath by solid–phase microextraction and gas chromatography–mass spectrometry. Microchem J 91:149–152
Gupta PK, Manral L, Ganesan K, Dubey DK (2007) Use of single–drop microextraction for determination of fentanyl in water samples. Anal Bioanal Chem 388:579–583
Van Nimmen NFJ, Poels KLC, Veulemans HAF (2004) Highly sensitive gas chromatographic—mass spectrometric screening method for the determination of picogram levels of fentanyl, sufentanil and alfentanil and their major metabolites in urine of opioid exposed workers. J Chromatogr B 804:375–387
Dufresne C, Favetta P, Gonin R, Bureau J, Guitton J (2002) Simultaneous determination of fentanyl and midazolam in plasma using direct solid–phase microextraction before gas chromatography–mass spectrometry analysis. Anal Lett 35:1575–1590
Huynh NH, Tyrefors N, Ekman L, Johansson M (2005) Determination of fentanyl in human plasma and fentanyl and norfentanyl in human urine using LC–MS/MS. J Pharm Biomed Anal 37:1095–1100
Ghazi-Khansari M, Zendehdel R, Pirali-Hamedani M, Amini M (2006) Determination of morphine in the plasma of addicts in using Zeolite Y extraction following high–performance liquid chromatography. Clin Chim Acta 364:235–238
Soriano C, Munoz-Guerra J, Carreras D, Rodriguez C, Rodriguez AF, Cortes R (1996) Automated analysis of drugs in urine. J Chromatogr B 687:183–187
Meadway C, George S, Braithwaite R (2002) A rapid GC–MS method for the determination of dihydrocodeine, codeine, norcodeine, morphine, normorphine and 6–MAM in urine. Forensic Sci Int 127:136–141
Kudo K, Ishida T, Hara K, Kashimura S, Tsuji A, Ikeda N (2007) Simultaneous determination of 13 amphetamine related drugs in human whole blood using an enhanced polymer column and gas chromatography–mass spectrometry. J Chromatogr B 855:115–120
Prosen H, Zupancíicí-Kralj L (1999) Solid–phase microextraction. Trends Anal Chem 18:272–282
Sarafraz-Yazdi A, Amiri A (2010) Liquid–phase microextraction. Trends Anal Chem 29:1–14
Barri T, Jönsson J-Å (2008) Advances and developments in membrane extraction for gas chromatography: Techniques and applications. J Chromatogr A 1186:16–38
Chen Y, Guo Z, Wang X, Qiu C (2008) Sample preparation. J Chromatogr A 1184:191–219
Pawliszyn J (1999) Applications of solid phase microextraction. Royal Society of Chemistry, Cambridge
Pedersen-Bjergaard S, Rasmussen KE (2008) Liquid–phase microextraction with porous hollow fibers, a miniaturized and highly flexible format for liquid–liquid extraction. J Chromatogr A 1184:132–142
Ridgway K, Lalljie SPD, Smith RM (2007) Sample preparation techniques for the determination of trace residues and contaminants in foods. J Chromatogr A 1153:36–53
Xu L, Basheer C, Lee HK (2007) Developments in single–drop microextraction. J Chromatogr A 1152:184–192
Pedersen-Bjergaard S, Rasmussen KE (1999) Liquid–liquid–liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal Chem 71:2650–2656
Psillakis E, Kalogerakis N (2003) Developments in liquid–phase microextraction. Trends Anal Chem 22:565–574
Rasmussen KE, Pedersen-Bjergaard S (2004) Developments in hollow fiber–based, liquid–phase microextraction. Trends Anal Chem 23:1–10
Lee J, Lee HK, Rasmussen KE, Pedersen-Bjergaard S (2008) Environmental and bioanalytical applications of hollow fiber membrane liquid–phase microextraction: a review. Anal Chim Acta 624:253–268
Saraji M, Farajmand B (2008) Application of single–drop microextraction combined with in–microvial derivatization for determination of acidic herbicides in water samples by gas chromatography–mass spectrometry. J Chromatogr A 1178:17–23
Saraji M, Mousavi F (2010) Use of hollow fiber–based liquid–liquid–liquid microextraction and high–performance liquid chromatography–diode array detection for the determination of phenolic acids in fruit juices. Food Chem 123:1310–1317
Xiao Q, Hu B, Yu C, Xia L, Jiang Z (2006) Optimization of a single–drop microextraction procedure for the determination of organophosphorus pesticides in water and fruit juice with gas chromatography–flame photometric detection. Talanta 69:848–855
De Jager LS, Andrews AR (2001) Development of a screening method for cocaine and cocaine metabolites in urine using solvent microextraction in conjunction with gas chromatography. J Chromatogr A 911:97–105
Li G, Zhang L, Zhang Z (2008) Determination of polychlorinated biphenyls in water using dynamic hollow fiber liquid–phase microextraction and gas chromatography–mass spectrometry. J Chromatogr A 1204:119–122
Xia L, Hu B, Wu Y (2007) Hollow fiber–based liquid–liquid–liquid microextraction combined with high–performance liquid chromatography for the speciation of organomercury. J Chromatogr A 1173:44–51
Pedersen-Bjergaard S, Rasmussen KE (2005) Bioanalysis of drugs by liquid–phase microextraction coupled to separation techniques. J Chromatogr B 817:3–12
Bagheri H, Es-haghi A, Khalilian F, Rouini MR (2007) Determination of fentanyl in human plasma by head–space solid–phase microextraction and gas chromatography–mass spectrometry. J Pharm Biomed Anal 43:1763–1768
Acknowledgments
The authors acknowledge Research Council of Isfahan University of Technology (IUT) and Center of Excellence in Sensor and Green Chemistry for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saraji, M., Boroujeni, M.K. Analysis of narcotic drugs in biological samples using hollow fiber liquid–phase microextraction and gas chromatography with nitrogen phosphorus detection. Microchim Acta 174, 159–166 (2011). https://doi.org/10.1007/s00604-011-0612-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0612-5