Abstract
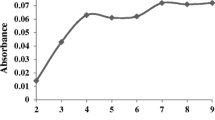
We report on a sensitive, reliable and relatively fast method for separation, preconcentration and determination of trace quantities of copper(II) ion. It is making use of nanometer-sized γ-alumina nanoparticles modified with sodium dodecyl sulfate (SDS). The adsorptive potential was assessed via a Langmuir isotherm and the maximal sorption capacity was found to be 138 mg g-1. The effects of pH values, amount of ligand, flow rate, type of eluting agent, volume of eluent, and the volume of sample were examined. The effects of interfering ions on the recovery of the analyte were also investigated. Copper ion was then determined by flame atomic absorption spectrometry. The relative standard deviation for five replicate determinations (at 50 μg L−1 of copper) is 3.3%. The detection limit (at 3 s) is 2.5 μg L−1. This method was validated with a certified reference material of oyster tissue (NIST SRM 1566b) and the results coincided well with the certified values. The procedure was successfully applied to the determination of Cu in water and food samples.

Alumina nanoparticles modified with SDS have been used as sorbent for separation and preconcentration of copper after complexation with APDC.





Similar content being viewed by others
References
Ngeontae W, Aeungmaitrepirom W, Tuntulani T, Imyim A (2009) Highly selective preconcentration of Cu(II) from seawater and water samples using amidoamidoxime silica. Talanta 78:1004–1010
Fathi SAM, Parinejad M, Yaftian MR (2008) Multidentate nitrogen/oxygen donor ionophores; their use as selective extracting and mobile-carrier agents for copper(II) ions. Sep Purif Technol 64:1–7
Watling HR, Perrot FA, Shiers DW, Grosheva A, Richards TN (2009) Impact of the copper solvent extraction reagent LIX 984 N on the growth and activity of selected acidophiles. Hydrometallurgy 95:302–307
da Silva EL, Martins AO, Valentini A, de Favere VT, Carasek E (2004) Application of silica gel organofunctionalized with 3(1-imidazolyl)propyl in an on-line preconcentration system for the determination of copper by FAAS. Talanta 64:181–189
Ferreira HS, Santos ACN, Portugal LA, Costa ACS, Miro M, Ferreira SLC (2008) Pre-concentration procedure for determination of copper and zinc in food samples by sequential multi-element flame atomic absorption spectrometry. Talanta 77:73–76
Shokrollahi A, Ghaedi M, Hossaini O, Khanjari N, Soylak M (2008) Cloud point extraction and flame atomic absorption spectrometry combination for copper(II) ion in environmental and biological samples. J Hazard Mater 160:435–440
Rocha SAN, Dantas AF, Jaeger HV, Costa ACS, Leao ES, Goncalves MR (2008) Spectrofotometric determination of copper in sugar cane spirit using biquinoline in the presence of ethanol and Triton X-100. Spectrochimica Acta Part A 71:1414–1418
Goswami A, Singh AK (2002) Silica gel functionalized with resacetophenone: synthesis of a new chelating matrix and its application as metal ion collector for their flame atomic absorption spectrometric determination. Anal Chim Acta 454:229–240
Fritz JS (1999) Aanlytical solid-phase extraction. Wiley-VCH, New York
Zhuqing W, Min W, Genhua W, Yuyong S, Chiyang H (2010) Ion-imprinted sol–gel nanotubes membrane for selective separation of copper ion from aqueous solution. Microchim Acta 169:195–200
Wu G, Song G, Wu D, Shen Y, Wang Z, He C (2010) Synthesis of ion-imprinted mesoproprous silica gel sorbent for selective adsorption of copper ions in aqueous media. Mirochim Acta 171:203–209
Soylak M, Karatepe AU, Elci L, Dogan M (2003) Column preconcentration/sepration and atomic absorption spectrometric determinations of some heavy metals in tablet salt samples using Amberlite XAD-1180. Turk J Chem 27:235–242
Duran C, Gundogdu A, Bulut VN, Soylak M, Elci L, Senturk HB, Tufekci M (2007) Solid phase extraction of Mn(II), Co(II), Ni(II), Cu(II), Cd(II) and Pb(II) ions from environmental samples. J Hazard Mater 146:347–355
Soylak M, Dogan M (1996) Column preconcentration of trace amounts of copper on activated carbon from natural water samples. Anal Lett 29:635–642
Hiraide M, Hori J (1999) Enrichment of metal-APDC complexes on admicelle-coated alumina for water analysis. Anal Sci 15:1055–1058
Klabunde KJ (2001) Nanoscale materials in chemistry. Wiley, New York
Ramesh A, Devi BA, Hasegawa H, Maki T, Ueda K (2007) Nanometer-sized alumina coated with chromotropic acid as solid phase metal extractant from environmental samples and determination by inductively coupled plasma atomic emission spectrometry. Microchim J 86:124–130
Hashemi P, Bagheri S, Fat’hi MR (2005) Factorial design for optimization of experimental variables in preconcentration of copper by a chromotropic acid loaded Q- Sepharose adsorbent. Talanta 68:72–78
Afkhami A, Saber-Tehrani M, Bagheri H, Madrakian T (2011) Flame atomic absorption spectrometric determination of trace amounts of Pb(II) and Cr(III) in biological, food and environmental samples after preconcentration by modified nano-alumina. Microchim Acta 172:125–136
Afkhami A, Madrakian T, Ahmadi R, Bagheri H, Tabatabaee (2011) Chemically modified alumina naoparticles for selective solid phase extraction and preconcentration of trace amounts of Cd(II). Microchim Acta 175:69–77
Mahmoud ME (2002) Study of the selectivity characteristic incorporated into physically adsorbed alumina phases. II. mercaptonicotinic acid and potential applications as selective stationary phases for separation, extraction, and preconcentration of lead (II) and copper (II). J Liq Chrom Rel Technol 25:1187–1199
Pesavento M, Profumo A, Alberti G, Conti F (2003) Adsorption of lead(II) and copper(II) on activated carbon by complexation with surface functional groups. Anal Chim Acta 480:171–180
Kendüzler E, Türker AR (2003) Atomic absorption spectrophotometric determination of trace copper in waters, aluminium foil and tea samples after preconcentration with 1-nitroso-2-naphthol-3,6-disulfonic acid on Ambersorb 572. Anal Chimica Acta 480:259–266
Birlik E, Ersöz A, Denizli A, Say R (2006) Preconcentration of copper using double-imprinted polymer via solid phase extraction. Anal Chim Acta 565:145–151
Xie F, Lin X, Wu X (2008) Solid phase extraction of lead (II), copper (II), cadmium (II) and nickel (II) using gallic acid-modified silica gel prior to determination by flame atomic absorption spectrometry. Talanta 74:836–843
Yin J, Jiang Z, Chang G, Hu B (2005) Simultaneous on-line preconcentration and determination of trace metals in environmental samples by flow injection combined with inductively coupled plasma mass spectrometry using a nanometer-sized alumina packed micro-column. Anal Chim Acta 540:333–339
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manzoori, J.L., Amjadi, M. & Darvishnejad, M. Separation and preconcentration of trace quantities of copper ion using modified alumina nanoparticles, and its determination by flame atomic absorption spectrometry. Microchim Acta 176, 437–443 (2012). https://doi.org/10.1007/s00604-011-0738-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0738-5