Abstract
Purpose
Only a third of children with cancer and febrile neutropenia (FN) have a proven bacterial infection; nevertheless, most children are hospitalized and treated with intravenous antibiotics. Several biomarkers have been proposed as predictive markers for bacterial infection in this population. We aimed to evaluate the role of interleukin-6 (IL-6) and procalcitonin (PCT) in diagnosing bacterial infection in children with cancer and FN.
Methods
The study population was derived from a prospective database (2006–2013, IL-8 study) comprising children with cancer who presented with FN. From stored plasma samples (taken at admission and/or at 12–24 h), we determined the PCT and IL-6 levels. Consequently, we explored their relation with the presence of bacterial infection (positive blood culture, radiologically documented infection or clinical bacterial focus). We predefined cutoff values at 60 ng/L for IL-6 and 0.25 ng/mL for PCT.
Results
Seventy-seven FN episodes in 55 children with cancer were included. In 18 episodes (23.4%), a bacterial infection was documented. Both at presentation and after 12–24 h, median values of IL-6 and PCT were significantly higher in patients with a bacterial infection compared to patients without a bacterial infection. With both biomarkers above cutoff values, sensitivity was 93% (with either one, this was even 100%). The identified group at low risk for bacterial infection comprised 41% of the population.
Conclusion
PCT and IL-6 are promising markers in identifying bacterial infection in children with cancer and FN. In a subsequent project, we will incorporate these biomarkers in a risk assessment model that we will test prospectively in a clinical trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children with cancer who develop fever after receiving chemotherapy are usually immediately referred to the hospital. In case their neutrophil count is very low, all patients used to be hospitalized and treated with broad-spectrum intravenous antibiotics until they become afebrile and neutrophil counts are starting to recover [1]. This aggressive strategy to manage febrile neutropenia has led to a great decrease in infection-related mortality and morbidity [2, 3]. On the other hand, studies have shown that only 20–30% of children with febrile neutropenia have a proven bacterial infection [4, 5]. Other causes of fever during chemotherapy may be the effect of (chemotherapeutic) drugs, viral or fungal infections, blood transfusions, mucositis, or the malignancy itself [4]. In recent years, it has become practice to manage some patients with low risk fever and neutropenia as an outpatient with oral antibiotics. Being at low risk of systemic infection or sepsis, these patients benefit from a milder approach, preventing overtreatment [6, 7]. Yet, it is still very difficult to distinguish children with and without bacterial infection upon presentation, because at that time, fever may be the only symptom of bacterial infection [8]. Other symptoms are typically lacking, since the diminished number of neutrophils causes an impaired inflammatory response [8, 9].
Various risk assessment strategies have previously been proposed to rapidly determine whether a patient is at low or high risk for bacterial infection at the moment of presentation with febrile neutropenia. However, each risk assessment model has only been validated for its specific hospital setting, region, and patient population. There is no international consensus or protocol with regard to the appropriate strategy to use yet [1, 10].
Previously, we have shown that it is safe to withhold antibiotics in selected cancer patients who are at low risk of bacterial infection, based on a risk assessment model that included objective clinical parameters in combination with the infectious biomarker interleukin-8 (IL-8) [6]. In an additional multicenter study, we have demonstrated that it is also safe to shorten antibiotic treatment to 72 h in selected medium-risk patients, based on the same clinical criteria and IL-8 [4].
IL-6 and PCT have been proposed by some investigators to be just as good or even better than IL-8 at predicting bacterial infection [11,12,13,14]. In addition, implementation of IL-6 and PCT in the Netherlands might be more feasible, given the pragmatic reason that in several Dutch hospitals IL-8 measurement is seldomly performed and thus (1) not available 24-h a day and (2) is associated with high costs and long waiting time.
In this study, we have evaluated the role of IL-6 and PCT, separately and in combination, in diagnosing bacterial infection at the onset and 12–24 h after presentation with febrile neutropenia. We were particularly curious to find out if we can replace IL-8 by IL-6 and/or PCT in our original risk assessment model and hence improve the model with regard to sensitivity and specificity. With a better model, we might be able to withhold antibiotics in more patients and discharge children earlier, improving their quality of life.
Methods
Patients
This study was performed at the Department of Pediatric Oncology and Hematology at the University Medical Center of Groningen, the Netherlands. The study population was derived from the database of the prospective IL-8 study by Miedema et al. [4]. The inclusion criteria of this previous study were maintained in this study; all outpatient pediatric cancer patients that had presented with fever and chemotherapy-induced neutropenia between June 2006 and July 2013 were initially included. Body temperature was measured with a digital ear thermometer. Fever was invariably defined as a single body temperature > 38.5 or two or more recordings of > 38.0 during a period of 6 h. Neutropenia was defined as absolute neutrophil count (ANC) < 0.5 × 109/L or, if not available, leucocytes < 1.0 × 109/L. All patients had undergone a physical examination. Additionally, routine blood counts and diagnostic blood and other appropriate cultures or diagnostic tests had been performed.
Plasma samples remaining after measurement of serum levels of IL-8 had been stored for future research. In this study, we determined plasma values of IL-6 and PCT on admission (T0) and after 12–24 h (T1). Patients were excluded from this study when no or not enough plasma was available to perform laboratory tests for IL-6 and PCT at T0 and/or T1. Patients who had received antibiotics other than the usual prophylactic antibiotic treatment strategies or had undergone allogeneic stem cell transplantation in the previous month had already been excluded from the IL-8 study [4]. Medical records of all the patients included in this study were reviewed to complete missing data (e.g., x-thorax reports). Only the IL-8 values of the patients included in this particular study were extracted from the IL-8 database and included for proper comparison with IL-6 and PCT values.
The institutional review board approved the IL-8 study protocol. The study was registered at www.trialregister.nl, trial ID number NTR3165. All patients and/or their parents, dependent on age, have given informed consent for participation and storage of serum samples for future research [4].
Laboratory tests
Plasma samples had been stored at − 80 °C in EDTA tubes. Plasma IL-6 values were measured in nanograms per liter using an enzyme-linked immunosorbent assay (ELISA Elecsys IL-6) assay. Plasma PCT levels were determined by an enzyme-linked fluorescent immunoassay (VIDAS® B.R.A.H.M.S. PCT™, provided by bioMérieux) and displayed in nanograms per milliliter. The lower detection limit was 0.05 ng/mL. Cutoff values were based on our previous research and literature findings and set at 60 ng/L and 0.25 ng/mL for IL-6 and PCT, respectively [6, 14, 15].
Group definitions
Patients were divided into two groups: with and without bacterial infection. Bacterial infection was defined as either microbiologically documented infection (a blood culture or culture of fluid collected from an otherwise sterile site positive for a bacterial pathogen) or radiologically documented infection (pneumonia or sinusitis). In other words, the bacterial infection group consists of patients that require antibiotics in any case.
Statistical analysis
Patient characteristics were summarized using descriptive statistics. Because data were not normally distributed, numerical data were expressed as medians with ranges. Accordingly, comparison between the two groups was performed using the nonparametric Mann-Whitney U test. Categorical data were compared using chi-square analysis. In all statistical tests, two-sided p values of < 0.05 were considered significant. All episodes, including repeated episodes in single patients, are treated as statistically independent. A sensitivity analysis, including only a patient’s first episode of febrile neutropenia, was performed to validate the accuracy of this decision rule. Receiver operating characteristics (ROC) curves were constructed for each biomarker at each time point. The area under the curve (AUC) was determined and cutoff values for the biomarkers were determined and compared to the literature. Possible cutoff values from ROC analyses were based on the Youden Index [15]. For the chosen cutoff values, the predictive performances (sensitivity, specificity, positive predictive value, and negative predictive value) with corresponding 95% confidence intervals (CIs) were calculated. CIs for sensitivity and specificity are exact Clopper-Pearson CIs. CIs for the predictive values are the standard logit CIs. Pearson correlation coefficients were used to investigate potential relationships between the biomarkers IL-8 and IL-6. Statistical analysis was performed using SPSS for Mac OS X version 24 (SPSS, IBM Company, Armonk, New York, USA). GraphPad Prism for Mac OS X version 7.0 (GraphPad Software, La Jolla, California, USA) was used to create graphics.
Results
Patient characteristics
One hundred sixty-five febrile neutropenic episodes were initially included in this study. Eighty-eight episodes were excluded, predominantly because no or not enough plasma sample was available for the determination of IL-6 and PCT. Thus, a total of 77 episodes of febrile neutropenia in 55 children with cancer were included in this study. These patients presented with febrile neutropenia between December 2008 and July 2013. Fifteen patients were enrolled more than once, with a maximum of five times. In 18 of the 77 episodes (23.4%), a bacterial infection was documented. Fourteen patients had positive blood cultures and two patients had positive cultures from another site (one patient with a perianal abscess and one with multiple furuncles). In two patients, pneumonia was radiographically confirmed.
To evaluate if the results from our selection of episodes were representative for the entire IL-8 cohort, we compared the characteristics of the excluded episodes (n = 88) and the included episodes (n = 77). There were no significant differences between groups with respect to sex, age, diagnosis, temperature, lab counts, and standard infection parameters.
The patient characteristics of the 77 included episodes and 55 children are shown in Table 1. With regard to sex, age, type of cancer, temperature, and blood counts on admission (Hb, leucocytes, neutrophils, and thrombocytes), no significant differences were found between the group with and the group without a bacterial infection. A difference in CRP levels on admission was observed, but this was not significant (median 96 mg/L in the bacterial infection group vs. 36 mg/L in the non-infectious group, p = 0.061). Serum values of IL-8 were significantly higher in the group with bacterial infection than in the group without bacterial infection, both on admission (176 vs. 54 ng/L) and after 12–24 h (218 vs. 45 ng/L) with p values of 0.002 and < 0.001, respectively.
Biomarkers
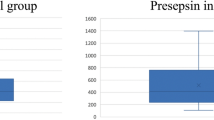
See Table 1 and Fig. 1. In 32 episodes, plasma samples were available both at presentation (T0) and 12–24 h later (T1), in 32 episodes plasma samples at T0 only, and in 13 episodes at T1 only. IL-6 levels were significantly higher in patients with bacterial infection compared with patients without bacterial infection both at T0 and T1 (345.2 vs. 91.9 ng/L, p = 0.003 and 242 vs. 57 ng/L, p = 0.061). PCT levels were significantly elevated in patients with bacterial infection compared with patients without bacterial infection at T0 (1.00 vs. 0.32 ng/mL, p = 0.021) and at T1 (1.80 vs. 0.38 ng/mL, p = 0.011), as well.
The discriminatory power of PCT and IL-6 markers for bacterial infection in patients with febrile neutropenia was evaluated using ROC curves, which are displayed in Fig. 2. For bacterial infection, the AUC demonstrated good discriminatory power for IL-6 on day 1 and day 2. On both time points, PCT performed was not as good as IL-6, with fair discriminatory power at T0 and poor discriminatory power at T1.
Table 2 shows the performance of IL-6 and PCT in terms of sensitivity and specificity at T0 and T1, at cutoff values of 60 ng/L for IL-6 and 0.25 ng/ml for PCT. These cutoff values were pre-chosen based on earlier research. The predefined cutoff values were found to be accurate according to the ROC analyses, based on the Youden Index and clinical relevance. IL-6 showed 100% sensitivity at T0 and T1; for PCT, this was 93.3 and 90% respectively. Values of IL-6 and IL-8 on both T0 and T1 correlated significantly (Pearson correlation coefficient 0.776, p < 0.001 for admission and 0.958, p < 0.001 after 12–24 h).
The analyses were continued by constructing combinations between IL-6 and PCT and evaluating their values in distinguishing bacterial infections from other causes of fever. Table 3 shows the p values, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of these combinations. With either one of the biomarkers above the cutoff value, the sensitivity is 100%, both at T0 and T1. Thus, all patients with a bacterial infection were identified in this study population. However, the specificity was low, identifying a low risk group of only 12 of 64 patients (19%) on admission and 9 of 45 patients (20%) after 12–24 h. Stating that both biomarkers had to be above the cutoff value, more patients could be identified at low risk, namely 26 of 64 patients (41%) at T0 and 23 of 45 patients (51%) at T1. However, one patient with a bacterial infection (a 13-year-old boy with a perianal abscess) had a serum PCT level below the cutoff value at both time points and consequently would have been failed to notice using these criteria.
Repeated episodes were treated as statistically independent in all the aforementioned analyses. A sensitivity analysis (with only the first episode per patient included) was performed to validate this approach. This showed similar results with the same statistical significance.
Discussion
In this study, we have investigated the diagnostic value of various biomarkers for predicting bacterial infection in children with cancer and febrile neutropenia. Our main aim was to identify patients that are at low risk for bacterial infection. We found that both IL-6 and PCT are promising in doing so. Both tests are highly sensitive, so the risk of missing a bacterial infection is low. Further study of (a combination) of these biomarkers might allow quick and safe identification of a low risk group.
Traditionally, pediatric cancer patients who present with febrile neutropenia are hospitalized and intravenous antibiotic therapy is initiated immediately and continued for as long as the neutropenia persists [1]. However, there is increasing evidence that this aggressive management is excessive to apply to all cases of febrile neutropenia (e.g., those with a viral infection). [16, 17] A diagnostic test that can distinguish between the group of patients that can benefit from a milder approach and patients at high risk for bacterial infection at the time of presentation would thus be of great value.
Lehrnbecher et al. have emphasized this once more in the recently updated Guideline for the Management of Fever and Neutropenia in Children With Cancer [1]. Different research groups in various countries have proposed risk models based on a variety of biomarkers [2, 11,12,13, 16, 18,19,20] and clinical observations [3, 21,22,23]. Due to heterogeneity of these studies, it is currently not possible to extract a reliable, broadly applicable decision rule. In a previously performed large, prospective study in the Netherlands, we have introduced a risk model based on objective clinical parameters and the biomarker IL-8 [4]. With this model, we showed that it is feasible to distinguish a low risk group (20%) of the patients presenting with febrile neutropenia in clinical practice. We are planning a prospective study in which we want to be able to distinguish a larger proportion of patients with low risk of bacterial infection.
Therefore, as a first step, we have in this study analyzed the discriminatory power of PCT and IL-6, since these two biomarkers are suggested in multiple previous studies [2, 11,12,13,14,15,16, 18, 19, 24,25,26,27,28,29]. IL-6 is a cytokine with important effects on the development and differentiation of T and B lymphocytes and hematopoietic cells. On admission, IL-6 has demonstrated to be a sensitive marker for disease severity and it exhibits the potential to be a better early discriminator than CRP for children that will develop a serious infectious complication [2, 13, 19, 27,28,29,30]. PCT was first suggested as a biomarker for infection almost 25 years ago [16, 25]. PCT is a prehormone of calcitonin and serum levels of PCT increase in bacterial infection mainly due to the presence of bacterial endotoxins and exotoxins and inflammatory cytokines. PCT has been shown to reach plateau values within 8–24 h after the onset of a bacterial infection [16, 19, 24].
A second motivation for this study was the problems we encountered while introducing IL-8 in the clinical setting. Measurements of IL-8 are currently not routinely performed in our hospital laboratory, which makes it an expensive and impractical biomarker. In contrast, PCT and/or IL-6 measurements are available at low cost and around the clock. Using either or both of them would make the implementation of a risk model, if proven feasible, easier, and more cost-effective.
In this study, IL-6 was shown to be a highly sensitive biomarker. At a cutoff level of 60 ng/L, both at presentation and at 12–24 h after presentation, it does not miss a single high risk episode. In addition, L-6 values correlated well with IL-8 values, which supports the possibility of replacing IL-8 by IL-6 in a risk model. PCT was slightly less sensitive, missing one episode of a 13-year-old boy with a perianal abscess. In the clinical setting, biomarkers will always be used in addition to clinical factors such as a detailed patient history and a physical examination. Translating this to the aforementioned episode categorized as low risk according to two subsequent PCT measurements, this episode is unlikely to be regarded as low risk in clinical practice. We expect any treating physician would start antibiotic intravenous therapy regardless of laboratory values in the case of a perianal abscess. In this study, combining PCT and IL-6 led to the identification of an even larger group of low risk episodes (on admission 41%, as compared to 20% in the IL-8 study) [4].
When interpreting these findings, one should naturally take the limitations of this study into account. First, although the current study population and the majority of data were derived from a prospective database (IL-8 study), additional data were collected from medical records. This retrospective data is expected not to be as accurate or complete as prospectively collected data. Second, another disadvantage of being a follow-up study on a previous prospective trial is that not all plasma samples originally taken were available anymore. Some patients had to be excluded completely; of other patients, only a baseline or 12–24 h sample was available. This made it more difficult to explore changes over time. Group sizes at T1 have become small, which could be part of the reason for the non-significant difference between the group with and without bacterial infection while combining both biomarkers at this time point (Table 3). Third, we did not investigate viral or fungal infections. It would have been valuable to take other identifiable origins of fever than bacterial infections into account. Fourth and final, we did not take vital signs or other clinical criteria into account, as these were not retrievable from most patient records. As mentioned before, this can be of great interest when developing a risk model that combines biomarkers and clinical findings [10].
In conclusion, PCT and IL-6 might be good alternatives for IL-8 and CRP in identifying low risk febrile neutropenic episodes in children with cancer, especially when these biomarkers are combined. In the near future, we plan to elaborate on this work. First, we will perform a prospective validation study. Patients will receive standard care while clinical data and biomarkers at different times (e.g., on admission, after 24 h and after 48 h) are obtained. If the combination of biomarkers and clinical data is successful (i.e., no missed high risk episodes and identification of a significant low risk group), the next step would be a large, prospective, multinational trial in which we will use the identified optimal combination in a risk stratification model. With this, we aim to decrease unnecessary antibiotic use and hospital admission in children with cancer and febrile neutropenia, which has the potential to contribute greatly to the quality of life in these patients and their families.
References
Lehrnbecher T, Robinson P, Fisher B, Alexander S, Ammann RA, Beauchemin M, Carlesse F, Groll AH, Haeusler GM, Santolaya M, Steinbach WJ, Castagnola E, Davis BL, Dupuis LL, Gaur AH, Tissing WJE, Zaoutis T, Phillips R, Sung L (2017) Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J Clin Oncol 35:2082–2094. https://doi.org/10.1200/JCO.2016.71.7017
Stryjewski GR, Nylen ES, Bell MJ, Snider RH, Becker KL, Wu A, Lawlor C, Dalton H (2005) Interleukin-6, interleukin-8, and a rapid and sensitive assay for calcitonin precursors for the determination of bacterial sepsis in febrile neutropenic children. Pediatr Crit Care Med 6:129–135. https://doi.org/10.1097/01.PCC.0000149317.15274.48
Macher E, Dubos F, Garnier N, Delebarre M, de Berranger E, Thebaud E, Mazingue F, Leblond P, Martinot A (2010) Predicting the risk of severe bacterial infection in children with chemotherapy-induced febrile neutropenia. Pediatr Blood Cancer 55:662–667. https://doi.org/10.1002/pbc.22586
Miedema KGE, Tissing WJE, Abbink FCH, Ball LM, Michiels EMC, van Vliet MJ, de Vries WY, Kamps WA, Norbruis OF, Fiocco M, de Groot-Kruseman HA, van de Wetering MD, de Bont ESJM (2016) Risk-adapted approach for fever and neutropenia in paediatric cancer patients—a national multicentre study. Eur J Cancer 53:16–24. https://doi.org/10.1016/j.ejca.2015.10.065
Ammann RA, Hirt A, Lüthy AR, Aebi C (2003) Identification of children presenting with fever in chemotherapy-induced neutropenia at low risk for severe bacterial infection. Med Pediatr Oncol 41:436–443. https://doi.org/10.1002/mpo.10320
Oude Nijhuis C, Kamps WA, Daenen S et al (2005) Feasibility of withholding antibiotics in selected febrile neutropenic cancer patients. J Clin Oncol 23:7437–7444. https://doi.org/10.1200/JCO.2004.00.5264
Loeffen EA, te Poele EM, Tissing WJ, et al (2016) Very early discharge versus early discharge versus non-early discharge in children with cancer and febrile neutropenia. Cochrane Database Syst Rev https://doi.org/10.1002/14651858.CD008382.pub2
Sickles EA, Greene WH, Wiernik PH (1975) Clinical presentation of infection in granulocytopenic patients. Arch Intern Med 135:715–719. https://doi.org/10.1001/archinte.1975.00330050089015
Goldstein B, Giroir B, Randolph A (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6:2–8. https://doi.org/10.1097/01.PCC.0000149131.72248.E6
Phillips RS, Sung L, Amman RA et al (2016) Predicting microbiologically defined infection in febrile neutropenic episodes in children: global individual participant data multivariable meta-analysis. Br J Cancer 114:623–630. https://doi.org/10.1038/bjc.2016.28
Kitanovski L, Jazbec J, Hojker S, Derganc M (2014) Diagnostic accuracy of lipopolysaccharide-binding protein for predicting bacteremia/clinical sepsis in children with febrile neutropenia: comparison with interleukin-6, procalcitonin, and C-reactive protein. Support Care Cancer 22:269–277. https://doi.org/10.1007/s00520-013-1978-1
Aggarwal R, Bansal D, Bansal F et al (2013) Interleukin-5, interleukin-6, interleukin-8 and tumour necrosis factor-alpha levels obtained within 24-h of admission do not predict high-risk infection in children with febrile neutropenia. Ind J Med Microbiol 31:226–229. https://doi.org/10.4103/0255-0857.115624
Soker M, Çolpan L, Ece A, Deveciog C (2001) Serum levels of IL-1 beta, sIL-2R, IL-6, IL-8, and TNF-alpha in febrile children with cancer and neutropenia. Med Oncol 18:51–57. https://doi.org/10.1385/MO:18:1:51
Diepold M, Noellke P, Duffner U, Kontny U, Berner R (2008) Performance of Interleukin-6 and Interleukin-8 serum levels in pediatric oncology patients with neutropenia and fever for the assessment of low-risk. BMC Infect Dis 8:28. https://doi.org/10.1186/1471-2334-8-28
Fluss R, Faraggi D, Reiser B (2005) Estimation of the Youden Index and its associated cutoff point. Biom J 47(4):458–472
Miedema KGE, De Bont ESJM, Elferink RFMO et al (2011) The diagnostic value of CRP, IL-8, PCT, and sTREM-1 in the detection of bacterial infections in pediatric oncology patients with febrile neutropenia. Support Care Cancer 19:1593–1600. https://doi.org/10.1007/s00520-010-0987-6
Hodgson-Viden H, Grundy PE, Robinson JL et al (2005) Early discontinuation of intravenous antimicrobial therapy in pediatric oncology patients with febrile neutropenia. BMC Pediatr 5:10. https://doi.org/10.1186/1471-2431-5-10
Hitoglou-Hatzi S, Hatzistilianou M, Gougoustamou D, Rekliti A, Agguridaki C, Athanassiadou F, Frydas S, Kotsis A, Catriu D (2005) Serum adenosine deaminase and procalcitonin concentrations in neutropenic febrile children with acute lymphoblastic leukaemia. Clin Exp Med 5:60–65. https://doi.org/10.1007/s10238-005-0067-2
Fleischhack G, Kambeck I, Cipic D, Hasan C, Bode U (2008) Procalcitonin in paediatric cancer patients: its diagnostic relevance is superior to that of C-reactive protein, interleukin 6, interleukin 8, soluble interleukin 2 receptor and soluble tumour necrosis factor receptor II. Br J Haematol 111:1093–1102. https://doi.org/10.1111/j.1365-2141.2000.02458.x
EL-Maghraby SM, Moneer MM, Ismail MM et al (2007) The diagnostic value of C-reactive protein, interleukin-8, and monocyte chemotactic protein in risk stratification of febrile neutropenic children with hematologic malignancies. J Pediatr Hematol Oncol 29:131–136. https://doi.org/10.1097/MPH.0b013e3180308770
Agyeman P, Aebi C, Hirt A, Niggli FK, Nadal D, Simon A, Ozsahin H, Kontny U, Kühne T, Beck Popovic M, Leibundgut K, Bodmer N, Ammann RA (2011) Predicting bacteremia in children with cancer and fever in chemotherapy-induced neutropenia: results of the prospective multicenter SPOG 2003 FN study. Pediatr Infect Dis J 30:e114–e119. https://doi.org/10.1097/INF.0b013e318215a290
Santolaya ME, Alvarez AM, Avilés CL, Becker A, Cofré J, Enríquez N, O’Ryan M, Payá E, Salgado C, Silva P, Tordecilla J, Varas M, Villarroel M, Viviani T, Zubieta M (2002) Prospective evaluation of a model of prediction of invasive bacterial infection risk among children with cancer, fever, and neutropenia. Clin Infect Dis 35:678–683. https://doi.org/10.1086/342064
Delebarre M, Garnier N, Macher E, Thebaud E, Mazingue F, Leblond P, Duhamel A, Martinot A, Dubos F (2015) Which variables are useful for predicting severe infection in children with febrile neutropenia? J Pediatr Hematol Oncol 37:468–474. https://doi.org/10.1097/MPH.0000000000000440
Hatzistilianou M, Rekliti A, Athanassiadou F, Catriu D (2010) Procalcitonin as an early marker of bacterial infection in neutropenic febrile children with acute lymphoblastic leukemia. Inflamm Res 59:339–347. https://doi.org/10.1007/s00011-009-0100-0
Martinez-Albarran M, de Jesus Perez-Molina J, Gallegos-Castorena S et al (2009) Procalcitonin and C-reactive protein serum levels as markers of infection in a pediatric population with febrile neutropenia and cancer. Pediatr Hematol Oncol 26:414–425. https://doi.org/10.3109/08880010903044797
Secmeer G, Devrim I, Kara A, Ceyhan M, Cengiz B, Kutluk T, Buyukpamukcu M, Yetgin S, Tuncer M, Uludag AK, Tezer H, Yildirim I (2007) Role of procalcitonin and CRP in differentiating a stable from a deteriorating clinical course in pediatric febrile neutropenia. J Pediatr Hematol Oncol 29:107–111. https://doi.org/10.1097/MPH.0b013e3180320b5b
Chaudhary N, Kosaraju K, Bhat K, Bairy I, Borker A (2012) Significance of interleukin-6 (IL-6) and C-reactive protein (CRP) in children and young adults with febrile neutropenia during chemotherapy for cancer. J Pediatr Hematol / Oncol 34:617–623. https://doi.org/10.1097/MPH.0b013e3182677fc6
De Bont E, Vellenga E, Swaanenburg JCM et al (1999) Plasma IL-8 and IL-6 levels can be used to define a group with low risk of septicaemia among cancer patients with fever and neutropenia. Br J Haematol 107:375–380. https://doi.org/10.1046/j.1365-2141.1999.01707.x
Phillips RS, Wade R, Lehrnbecher T, Stewart LA, Sutton AJ (2012) Systematic review and meta-analysis of the value of initial biomarkers in predicting adverse outcome in febrile neutropenic episodes in children and young people with cancer. BMC Med 10:6. https://doi.org/10.1186/1741-7015-10-6
Kitanovski L, Jazbec J, Hojker S, Gubina M, Derganc M (2006) Diagnostic accuracy of procalcitonin and interleukin-6 values for predicting bacteremia and clinical sepsis in febrile neutropenic children with cancer. Eur J Clin Microbiol Infect Dis 25:413–415. https://doi.org/10.1007/s10096-006-0143-x
Acknowledgements
First and foremost, we would like to thank all patients (and their families) who participated in the IL-8 study. Also we would like to thank bioMérieux for providing the immunoassay to determine PCT levels (VIDAS® B.R.A.H.M.S. PCT™).
Financial support
For this study, no grant or funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest relevant to this article to disclose.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van der Galiën, H.T., Loeffen, E.A.H., Miedema, K.G.E. et al. Predictive value of PCT and IL-6 for bacterial infection in children with cancer and febrile neutropenia. Support Care Cancer 26, 3819–3826 (2018). https://doi.org/10.1007/s00520-018-4249-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4249-3