Abstract
Background
Febrile neutropenia (FNP) causes significant morbidity and mortality in children undergoing treatment for cancer. The development of clinical decision rules to help stratify risks in paediatric FNP patients and the use of inflammatory biomarkers to identify high risk patients is an area of recent research. This study aimed to assess if procalcitonin (PCT) levels could be used to help diagnose or exclude severe infection in children with cancer who present with febrile neutropenia, both as a single measurement and in addition to previously developed clinical decision rules.
Methods
This prospective cohort study of a diagnostic test included patients between birth and 18 years old admitted with febrile neutropenia to the Paediatric Oncology and Haematology Ward in Leeds between 1st October 2012 and 30th September 2013. Each admission with FNP was treated as a separate episode. Blood was taken for a procalcitonin level at admission with routine investigations. ‘R’ was used for statistical analysis. Likelihood ratios were calculated and multivariable logistic regression.
Results
Forty-eight episodes from 27 patients were included. PCT >2 ng/dL was strongly associated with increased risk of severe infection (likelihood ratio of 26 [95% CI 3.5, 190]). The data suggests that the clinical decision rules are largely ineffective at risk stratification, frequently over-stating the risk of individual episodes. High procalcitonin levels on admission are correlated with a greatly increased risk of severe infection.
Conclusions
This study does not show a definitive benefit in using PCT in FNP though it supports further research on its use. The benefit of novel biomarkers has not been proven and before introducing new tests for patients it is important their benefit above existing features is proven, particularly due to the increasing importance of health economics.
Similar content being viewed by others
Background
Febrile neutropenia (FNP) is the clinical situation of raised temperature in the face of a low granulocyte count following anticancer therapy, indicating a risk of life-threatening infection. It remains a cause of significant morbidity and mortality [1]. While a traditional approach is to manage all cases with prolonged courses of in-hospital intravenous antibiotics, the development of clinical decision rules to help stratify risks in paediatric FNP patients has been an area of recent research [2].
The use of inflammatory biomarkers markers to identify high-risk patients with febrile neutropenia continues to be explored [3]. Procalcitonin (PCT) may be better than C-reactive protein (CRP) in helping identify patients with severe infection as the cause of temperature in neutropenia [4, 5]. In patients with FNP significantly higher PCT levels have been shown in bacteraemias, particularly gram negative infections, compared to viral illness or fever of unknown origin. It is claimed that PCT is not significantly raised in inflammatory conditions or mucositis [6]. However, other studies have shown no significant difference in PCT levels in bacterial infections. Meta-analysis shows significant heterogeneity between studies and further research is needed to assess if procalcitonin is clinically useful [3].
The aim of this study was to assess if procalcitonin levels can be used to help diagnose or exclude severe infection in children with cancer who present with febrile neutropenia, both as a single measurement and assessing it’s additional value of PCT above previously developed clinical decision rules.
Methods
A prospective cohort of children aged between birth and 18 years old who were undergoing anti-cancer treatment under the care of the paediatric oncology and haematology department at Leeds Teaching Hospitals, who consented and were admitted to the paediatric oncology and haematology ward in Leeds with FNP between 1st October 2012 and 30th September 2013 were included. Febrile neutropenia was defined, as per the Leeds guidelines, as two temperatures of more than 38.0 °C or one temperature of more than or equal to 38.5 °C and neutrophil count of less than 0.75 109/L, in the absence of an already-microbiologically documented infection. The neutrophil count is reported as the sum of the mature and immature band forms of neutrophils. All patients were routinely examined, admitted, and given broad-spectrum antibiotics as per the unit policy. Patients were excluded if they were not neutropenic. Each admission with FNP was treated as a separate episode. Consent was taken for children to participate from parents or guardians prior to presentation with FNP.
Additional blood for PCT was taken with admission (day 1) blood tests for FNP. Further samples for PCT were taken on day 2 and day 3 of admission for some patients. PCT samples were analysed at the end of the study period following data collection of clinical features and diagnosis for each episode of febrile neutropenia. PCT testing was done using a Siemens Advia Centaur XP. Using the classification system from the international PICNICC (Predicting Infectious Complications of Neutropenic sepsis in Children with Cancer) group each episode was classified into severe or non-severe infection (Fig. 1) [3].
‘R’ was used for statistical analysis. PCT values were divided into low (<0.5 ng/ml), intermediate (0.5 to 2 ng/ml) and high (>2 ng/ml) groups and after log-transformation of the value as a continuous variable [7–9]. Analysis of PCT in addition to clinical decision rules was undertaken by multivariable logistic regression. The null hypothesis was there would be no improvement in diagnostic accuracy with the addition of PCT to the previously developed clinical decision rules, with conventional significance defined as p < 0.05.
Ethical approval was given by Leeds (West) Research Ethics Committee (REC reference: 12/YH0376). Funding was provided by Candlelighter’s charity, who had no influence over the study, the analysis or the decision to publish. STROBE guidelines for cohort studies were adhered to [10].
Results
The cohort consisted of 48 episodes from 27 patients, with a median age 5 years 2 months (range 1y3m to 18y3m). Their diagnoses are in Table 1. Table 2 demonstrates the distribution of episodes per patient and samples per day of admission.
Assessed as a categorical variable, a high PCT value (>2 ng/dL) was strongly associated with risk of severe infection of 26 [95% CI 3.5, 190], with unclear associations with intermediate and low levels (See Table 3).
The additional value of procalcitonin levels, to previously developed clinical decision rules both as categorical values and as a continuous variable, was assessed. The rules were: the departmental guidelines for FNP treatment, Ammann rule [11], Rackoff rule [12], SPOG rule [13], Alexander rule [14] and PINDA rule [15]. The additional values of procalcitonin are shown as continuous (see Table 4) and categorical (see Table 5) variables.
These data suggest that the clinical decision rules are largely ineffective at risk stratification, frequently over-stating the risk of individual episodes. High procalcitonin levels on admission are correlated with a greatly increased risk of severe infection.
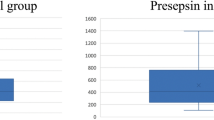
There is no evidence of correlation between PCT and Neutrophil count (r = −0.08). Insufficient data were collected to statistically assess the value of repeated measurements of PCT over time. The values are graphically displayed in Fig. 2, for the 25 episodes where data on more than one day were collected.
Discussion
This study supports the hypothesis that procalcitonin values measured on admission help identify those children who will develop a severe infection (as defined in Fig. 1) during their episode of FNP. Uncertainty remains as to the extent of this predictive ability, and if multiple-day testing can improve this further. There were no episodes of confirmed invasive fungal infection in this study so no direct conclusion about the value of PCT in fungal infection could be drawn.
There are seven previous studies [3, 7–9, 16–18] that have looked at PCT in FNP in children with cancer. The studies include between 29 to 278 patients, with most less than 100 patients, and 39 to 566 episodes. The results of these studies are presented in different ways. The PCT cut off values in the studies varied between 0.5 ng/dL and 2 ng/dL. Three studies that presented sensitivity and specificity all had sensitivities between 93 and 96.5% for FNP and severe infection. The specificity was between 70.6 and 97% [7–9]. The high sensitivities may be due to cut off values for procalcitonin being chosen to maximize the value of PCT following analysis of the data instead of prior to analysis as in this study.
The data collected was used to test existing clinical decision rules. Only in the PINDA and Amman rules low risk groups and the departmental guideline intermediate risk group were the odds of severe infection lower compared to the high risk group. Little validation of clinical decision rules have been done outside of their original dataset so information on how they perform in different patient groups is important.
No other currently published research study has looked at the use of PCT on admission in addition to clinical and laboratory features used in clinical decision rules. Single tests are rarely used to make decisions and it is important to see if new diagnostic tests are of additional benefit to features already used before they become part of routine use.
Multiple day testing has been previously examined in two studies. Santolaya et al. [17] showed that PCT levels did not discriminate between severe sepsis and non-severe infection at admission but did at day two. Stryjewski et al. [18] also showed no association with PCT levels and sepsis at admission but did show an association at 24 and 48 h. Only limited data was available for PCT on day two and day three of admission in this study. Although the PCT levels rose higher on day two in three out of four cases of severe infection compared to those cases with non-severe infection the significance is uncertain.
Often young adults are not included in studies, which limits how the results can be applied, but this study included a wide age range. The PCT values were not known until after data was collected which avoided clinician bias in interpreting the clinical features in light of the PCT result. As the cut off values were defined prior to collecting the data this avoided overestimation of the accuracy of PCT, which may have occurred in other studies who defined cut off values based on their own data.
Conclusions
This study does not provide conclusive evidence as to the value, or lack of value, of PCT in episodes of FNP with and without significant adverse outcomes though it supports further research on its use in association with clinical decision rules to identify patients at high risk and low risk of severe infection to help target appropriate management. Further analysis on larger datasets of the additional benefit of biomarkers to existing clinical and laboratory features used is an important step. The benefit of novel biomarkers has not been proven and before introducing new tests for patients it is important their benefit above existing features is proven, particularly due to the increasing importance of rational diagnostic testing and “choosing wisely” [19].
Abbreviations
- 95% CI:
-
95% confidence interval
- CMV:
-
Cytomegalovirus
- CRP:
-
C-reactive protein
- CSF:
-
Cerebrospinal fluid
- CVC:
-
Central venous catheter
- EORTC:
-
European Organisation for Research and Treatment of Cancer
- FNP:
-
Febrile neutropenia
- HSV:
-
Herpes simplex virus
- LR:
-
Likelihood ratio
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- PCT:
-
Procalcitonin
- PICNICC:
-
Predicting Infectious Complications of Neutropenic sepsis in Children with Cancer
- PICU:
-
Paediatric intensive care unit
- VZV:
-
Varicella zoster virus
References
Phillips B, Selwood K, Lane SM, Skinner R, Gibson F, Chisholm JC. Variation in policies for the management of febrile neutropenia in United Kingdom Children’s Cancer Study Group centres. Arch Dis Child. 2007;92:495–8.
Phillips B, Lehrnbecher T, Alexander S, Sung L. Updated systematic review and meta-analysis of the performance of risk prediction rules in children and young people with febrile neutropenia. PLoS One. 2012;7:1–9.
Phillips RS, Wade R, Lehrnbecher T, Stewart LA, Sutton AJ. Systematic review and meta-analysis of the value of initial biomarkers in predicting adverse outcome in febrile neutropenic episodes in children and young people with cancer. BMC Medicine. 2012;10:6. DOI: 10.1186/1741-7015-10-6. http://bmcmedicine.biomedcentral.com/articles/10.1186/1741-7015-10-6.
Brack E, Bodmer N, Simon A, et al. First-day step-down to oral outpatient treatment versus continued standard treatment in children with cancer and low-risk fever in neutropenia. A randomized controlled trial within the multicenter SPOG 2003 FN study. Pediatr Blood Cancer. 2012;59:423–30.
Kera WV. Risk assessment and treatment of low-risk patients with febrile neutropenia. Clin Infect Dis. 2006;42:633–40.
Delevaux I, Andre M, Colombier M, et al. Can procalcitonin measurement help in differentiatin between bacterial infection and other kinds of inflammatory processes? Ann Rheum Dis. 2003;62:337–40.
Hatzistilianou M, Rekleity A, Athanassiou K, DeLutiis MA, Conti P, Catriu D. Serial procalcitonin responses in infection of children with secondary immunodeficiency. Clin Invest Med. 2007;30:E75–85.
Hitoglou-Hatzi S, Hatzistilianou M, Gougoustamou D, et al. Serum adenosine deaminase and procalcitonin concentrations in neutropenic febrile children with acute lymphoblastic leukaemia. Clin Exp Med. 2005;5:60–5.
Kitanovski L, Jazbec J, Hojker S, Gubina M, Derganc M. Diagnostic accuracy of procalcitonin and interleukin-6 values for predicting bacteremia and clinical sepsis in febrile neutropenix children with cancer. Eur J Clin Microbiology Infect Dis. 2006;25:413–5.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296.
Ammann RA, Hirt A, Luthy AR, Aebi C. Identification of children presenting with fever in chemotherpay-induced neutropenia at low risk for severe bacterial infection. Med Pediatr Oncol. 2003;41:436–43.
Rackoff WR, Gonin R, Robinson C, Kreissman SG, Breitfeld PP. Predicting the risk of bacteremia in children with fever and neutropenia. J Clin Oncol. 1996;14:919–24.
Ammann RA, Bodmer N, Hirt A, et al. Predicting adverse events in children with fever and chemotherapy-induced neutropenia: the prospective multicenter SPOG 2003 FN study. J Clin Oncol. 2010;28:2008–14.
Alexander SW, Wade KC, Hibberd PL, Parsons SK. Evaluation of risk prediction criteria for episodes of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2002;24:38–42.
Santolaya ME, Alvarez AM, Becker A, et al. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. J Clin Oncol. 2001;19:3415–21.
Barnes C, Ignjatovic V, Newall F, et al. Change in serum procalcitonin (PCT) predicts the clinical outcome of children admitted with febrile neutropenia. Br J Haematol. 2002;118:1197.
Santolaya ME, Alvarez AM, Aviles CL, et al. Predictors of severe sepsis not clinically apparent during the first twenty-four hours of hospitalization in children with cancer, neutropenia, and fever. Pediatr Infect Dis. 2008;27:538–43.
Stryjewski GR, Nylen ES, Bell MJ, et al. Interleukin-6, Interleukin-8, and a rapid sensitive assay for calcitonin precursors for the determination of bacterial sepsis in febrile neutropenic children. Pediatr Crit Care Med. 2005;6:129–35.
Malhotra A, Maughan D, Ansell J, et al. Choosing wisely in the UK: the Academy of Medical Royal Colleges’ initiative to reduce the harms of too much medicine. Br Med J. 2015;350:h2308.
Acknowledgements
Research biochemistry department at Leeds General Infirmary for analyzing procalcitonin blood samples. Paediatric oncology research nurses at Leeds General Infirmary helped recruit and consent patients. Funding was provided by Candlelighter’s charity, who had no influence over the study, the analysis or the decision to publish.
Funding
Funding for the study was provided by Candlelighter’s Charity. They have had no influence over the conduct, analysis or decision to report the study.
Availability of data and materials
Data analysed during this study is included in this published article and its supplementary information files. The dataset used during the current study is available from the corresponding author on reasonable request (Additional file 1).
Authors’ contributions
VH participated in the design of study, acquisition of data, analysis and interpretation of the data, and drafted the manuscript. ADJ participated in acquisition of data and completed the STROBE checklist. GS participated in the design of the study. BP participated in the conception and design of the study, acquisition of data, analysis and interpretation of the data, and drafted the manuscript. All authors have reviewed the manuscript and agreed the final version.
Competing interests
Funding for the study was provided by Candlelighter’s Charity. They have had no influence over the conduct, analysis or decision to report the study. There are no other financial or non-financial conflicts of interest to report.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval was given by Leeds (West) Research Ethics Committee (REC reference: 12/YH0376). Consent was obtained from parents or guardians of all participants prior to inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Procalcitonin study data. (XLSX 65 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hemming, V., Jakes, A.D., Shenton, G. et al. Prospective cohort study of procalcitonin levels in children with cancer presenting with febrile neutropenia. BMC Pediatr 17, 2 (2017). https://doi.org/10.1186/s12887-016-0766-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-016-0766-8