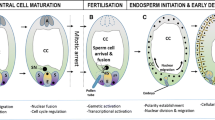
Abstract
Precocious seed development is usually prevented by a series of mechanisms that ensure seed production results from double fertilization. These events are circumvented in natural apomictic plant species that reproduce clonally through seed. Recent advances in molecular genetics using mutagenic approaches in model sexual plant species, such as Arabidopsis and Zea mays, have revealed some of the mechanisms that prevent such precocious seed development. An understanding of these mechanisms may lead to the development of techniques that will allow future crop plant species exhibiting hybrid vigor to be engineered such that their complex genomes can be fixed indefinitely, thereby maintaining high yields. Our current understanding of the mechanisms underlying the processes of reproductive development is discussed in this review.


Similar content being viewed by others
References
Antoine AF, Dumas C, Faure J-E, Feijó JA, Rougier M (2001) Egg activation in flowering plants. Sex Plant Reprod 14:21–26
Antoine AF, Faure J-E, Cordeiro S, Dumas C, Rougier M, Feijó JA (2000) A calcium influx is triggered and propagates in the zygote as a wavefront during in vitro fertilization of flowering plants. Proc Natl Acad Sci USA 97:10643–48
Asker SE, Hagberg A, Hagberg G (1983) Apomixis in barley? Sver Utsädesforen Tidskr 93:75–76
Balzer H-J, Borisiuk L, Meyer H-M, Matzk F, Bäumlein (1996) A pollen allergen-encoding gene is expressed in wheat ovaries. Plant Mol Biol 32:435–445
Baroux B, Blanvillain R, Gallois P (2001) Paternally inherited transgenes are down-regulated but retain low activity during early embryogenesis in Arabidopsis. FEBS Lett 509:11–16
Baroux C, Gagliardini V, Page DR, Grossniklaus U (2006) Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev 20:1081–1086
Baroux C, Pien S, Grossniklaus U (2007) Chromatin modification and remodeling during early seed development. Curr Opin Genet Dev doi:10.1016/j.gde.2007.09.004
Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C (2004) Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427:164–167
Berger F, Grini PE, Schnittger A (2006) Endosperm an integrator of seed growth and development. Curr Opin Plant Biol 9:664–670
Birchler JA (1993) Dosage analysis of maize endosperm development. Annu Rev Genet 27: 181–204
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AA, Miki BL, Custers JB, van Lookeren Campagne MM (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
Brand L, Horler M, Nuesch E, Vassalli S, Barrell P, Yang W, Jefferson RA, Grossniklaus U, Curtis MD (2006) A versatile and reliable two-component system for tissue-specific gene induction in Arabidopsis. Plant Physiol 141:1194–1204
Chase S (1969) Monoploids and monoploid-derivative of maize (Zea mays L.). Bot Rev 35:117–167
Casson S, Spencer M, Walker K, Lindsey K (2005) Laser capture microdissection for the analysis of gene expression during embryogenesis of Arabidopsis. Plant J 42:111–123
Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ (1997) Fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA 94:4223–4228
Choi Y, Gehring M, Johnson L, Hannon M, Harada JJ, Goldberg RB, Jacobsen SE, Fischer RL (2002) DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell 110:33–42
Choi Y, Harada JJ, Goldberg RB, Fischer RL (2004) An invariant aspartic acid in the DNA glycosylase domain of DEMETER is necessary for transcriptional activation of the imprinted MEDEA gene. Proc Natl Acad Sci USA 101:7481–6
Coe EH (1959) A line of maize with high haploid frequency. Am Nat 93:381–382
Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V (2002) Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111:185–196
Day RC, Grossniklaus U, Macknight RC (2005) Be more specific! Laser-assisted microdissection of plant cells. Trends Plant Sci 10:397–406
Deimling S, Röber F, Geiger HH (1997) Methodik ung genetic der in-vivo-haploidenduktion bei mais. Vortr Pflanzenzücht 38:203–224
Dilkes BP, Comai L (2004) A differential dosage hypothesis for parental effects in seed development. Plant Cell 16:3174–3180
Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U (2007) The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317:606–607
Evans MMS (2007) The indeterminate gametophyte1 gene of maize encodes a LOB domain protein required for embryo sac and leaf development. Plant Cell 19:46–62
Feil R, Berger F (2007) Convergent evolution of genomic imprinting in plants and mammals. Trends Genet 23:192–9
Gallois J-L, Nora FR, Mizukami Y, Sablowski R (2004) WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev 18:375–380
Gehring M, Huh JH, Hsieh TF, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL (2006) Demeter DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell 124:495–506
Gendrel A-V, Lippman Z, Yordan C, Colot V, Martienssen RA (2002) Dependence of heterochromatic histone H3 methylation patterns on the Arabidopsis gene DDM1. Science 297:1871–1873
Gernand D, Rutten T, Varshney A, Rubtsova M, Prodanovic S, Brüss C, Kumlehn J, Matzk F, Houben A (2005) Uniparental chromosome elimination at mitosis and interphase in wheat and pearl millet crosses involves micronucleus formation, progressive heterochromatinization, and DNA fragmentation. Plant Cell 17:2431–2438
Grimanelli D, Garcia M, Kaszas E, Perotti E, Leblanc O (2003) Heterochronic expression of sexual reproductive programs during apomictic development in Tripsacum. Genetics 165:1521–1531
Grimanelli D, Hernandez M, Perotti E, Savidan Y (1997) Dosage effects in the endosperm of diplosporous apomictic Tripsacum (Poaceae). Sex Plant Reprod 10:279–282
Grimanelli D, Leblanc O, Perotti E, Grossniklaus U (2001) Developmental genetics of gametophytic apomixis. Trends Genet 17:597–604
Grimanelli D, Perotti E, Ramirez J, Leblanc O (2005) Timing of the maternal-to-zygotic transition during early seed development in maize. Plant Cell 17:1061–1072
Grossniklaus U (2005) Genomic imprinting in plants: a predominantly maternal affair. In: Meyer P (eds) Annual plant reviews: plant epigenetics. Blackwell, Sheffield, pp. 174–200
Grossniklaus U, Paro R (2007) Transcriptional silencing by Polycomb group proteins. In: Allis CD, Jenuwein T, Reinberg D, Caparros M (eds) Epigenetics. Cold Spring Harbor, New York, pp. 211–230
Grossniklaus U, Spillane C, Page DR, Kohler C (2001) Genomic imprinting and seed development: endosperm formation with and without sex. Curr Opin Plant Biol 4:21–27
Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB (1998a) Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science 280:446–450
Grossniklaus U, Moore J, Gagliano W (1998b) Molecular and genetic approaches to understanding and engineering apomixes: Arabidopsis as a powerful tool. In: Virmani SS, Siddiq EA, Muralidharan K (eds) Advances in hybrid rice technology: Proc 3rd Int. hybrid rice symp. Manila, IRRI, pp 187–212
Golden TA, Schauer SE, Lang JD, Pien S, Mushegian AR, Grossniklaus U, Meinke DW, Ray A (2002) SHORT INTEGUMENTS1/SUSPENSOR1/CARPEL FACTORY, a Dicer homolog, is a maternal effect gene required for embryo development in Arabidopsis. Plant Physiol 130:808–822
Guitton AE, Page DR, Chambrier P, Lionnet C, Faure JE, Grossniklaus U, Berger F (2004) Identification of new members of fertilisation independent seed polycomb group pathway involved in the control of seed development in Arabidopsis thaliana. Development 131:2971–2981
Guitton E-A, Berger F (2005) Loss of function of MULTICOPY SUPPRESSOR OF IRA 1 produces nonviable parthenogenetic embryos in Arabidopsis. Curr Biol 15:750–754
Hagberg A, Hagberg G (1980) High frequency of spontaneous haploids in the progeny of an induced mutation in barley. Hereditas 93:341–343
Haig D, Westoby M (1989) Parent-specific gene expression and the triploid endosperm. Am Nat 134:147–155
Haig D, Westoby M (1991) Genomic imprinting in endosperm: its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications for the evolution of apomixis. Philos Trans R Soc London Ser B333:1–13
Hecht V, Vielle-Calzada J-P, Hartog MV, Schmidt EDL, Boutilier K, Grossniklaus U, de Vries SC (2001) The Arabidopsis somatic embryogenesis receptor kinase 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816
Hennig L, Taranto P, Walser M, Schönrock N, Gruissem W (2003) Arabidopsis MSI1 is required for epigenetic maintenance of reproductive development. Development 130:2555–2565
Huck N, Moore JM, Federer M, Grossniklaus U (2003) The female gametophytic control of pollen tube reception is disrupted in the Arabidopsis mutant feronia. Development 130:2149–2159
Husbands A, Bell EM, Shuai B, Smith HM, Springer PS (2007) LATERAL ORGAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res 35:6663–6671
Ikeda-Iwai M, Umehara M, Satoh S, Kamada H (2003) Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J 34:107–114
Jullien PE, Kinoshita T, Ohad N, Berger F (2006a) Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 18:1360–1372
Jullien PE, Katz A, Oliva M, Ohad N, Berger F (2006b) Polycomb group complexes self-regulate imprinting of the Polycomb group gene MEDEA in Arabidopsis. Curr Biol 16:486–492
Kaya H, Shibahara KI, Taoka KI, Iwabuchi M, Stillman B, Araki T (2001) FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104:131–42
Kermicle JL (1969) Androgenesis conditioned by a mutation in maize. Science 166:1422–1424
Kinoshita T, Miura A, Choi Y, Kinoshita Y, Cao X, Jacobsen SE, Fischer RL, Kakutani T (2004) One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303:521–523
Kinoshita T, Yadegari R, Harada JJ, Goldberg RB, Fischer RL (1999) Imprinting of the MEDEA Polycomb gene in the Arabidopsis endosperm. Plant Cell 11:1945–52
Köhler C, Grossniklaus U (2002) Epigenetic inheritance of expression states in plant development: the role of Polycomb group proteins. Curr Opin Cell Biol 14:773–779
Köhler C, Hennig L, Bouveret R, Gheyselinck J, Grossniklaus U, Gruissem W (2003a) Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. EMBO J 22:4804–4814
Köhler C, Hennig L, Spillane C, Pien S, Gruissem W, Grossniklaus U (2003b) The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes Dev 17:1540–1553
Köhler C, Page DR, Gagliardini V, Grossniklaus U (2005) The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet 37:28–30
Koltunow AM, Grossniklaus U (2003) Apomixis: a developmental perspective. Annu Rev Plant Biol 54:547–574
Koltunow AM, Johnson SD, Bicknell RA (1998) Sexual and apomictic development in Hieracium. Sex Plant Reprod 11:213–130
Kouzarides T (2002) Histone methylation in transcriptional control. Curr Opin Genet Dev 12:198–209
Kumlehn JK, Irik VC, Zihal A, Altschmied LM, Atzk FL, Örz H, Bäumlein H (2001) Parthenogenetic egg cells of wheat: cellular and molecular studies. Sex Plant Reprod 14:239–243
Lacadena J-R (1974) Spontaneous and induced parthenogenesis and androgenesis. In: Kasha KJ (ed) Haploids in higher plants. advances and potential. The University of Guelph, Guelph, pp 13–32
Leroy O, Hennig L, Breuninger H, Laux T, Köhler C (2007) Polycomb group proteins function in the female gametophyte to determine seed development in plants. Development 134:3639–3648
Lin B-Y (1978) Structural modifications of the female gametophyte associated with the indeterminate gametophyte (ig) mutant in maize. Can J Genet Cytol 20:249–257
Lin B-Y (1981) Megagametogenetic alterations associated with the indeterminate gametophyte (ig) mutant in maize. Rev Bras Biol 41:557–563
Lindroth AM, Shultis D, Jasencakova Z, Fuchs J, Johnson L, Schubert D, Patnaik D, Pradhan S, Goodrich J, Schubert I, Jenuwein T, Khorasanizadeh S, Jacobsen SE (2004) Dual histone H3 methylation marks at lysines 9 and 27 required for interaction with CHROMOMETHYLASE3. EMBO J 23:4286–4296
Lotan T, Ohto M, Yee KM, West MA, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93:1195–1205
Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM (1999) Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA 96:296–301
Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury AM (2000) Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc Natl Acad Sci U S A 97:10637–10642
Makarevich G, Leroy O, Akinci U, Schubert D, Clarenz O, Goodrich J, Grossniklaus U, Köhler C (2006) Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep 7:947–952
Matzk F, Meyer H-M, Bäumlein H, Balzer H-J, Schubert I (1995) A novel approach to the analysis of the initiation of embryo development in Gramineae. Sex plant Reprod 8:266–272
Matzk F (1996) The ‘Salmon system’ of wheat-a suitable model for apomixis research. Hereditas 125:299–301
Matzk F, Meyer H-M, Horstmann C, Balzer H-J, Bäumlein H, Schubert I (1997) A specific a-tubulin is associated with the initiation of. parthenogenesis in ‘Salmon’ wheat lines. Hereditas 126:219–224
Matzk F, Prodanovic S, Czihal A, Tiedemann J, Arzenton F, Blattner F, Kumlehn J, Altschmied L, Schubert I, Johnston A, Grossniklaus U, Bäumlein H (2007) Genetic control of apomixis: preliminary lessons from Poa, Hypericum and wheat egg cells. In: Horandl E, Grossniklaus U, van Dijk P, Sharbel TF (eds) Apomixis: evolution, mechanisms and perspectives. ARG Gantner Verlag, Rugell, Liechtenstein, pp 159–166
Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95:805–815
Meyer S, Scholten S (2007) Equivalent parental contribution to early plant zygotic development. Curr Biol 17:1686–91
Miyazaki S, Ito M (2006) Calcium signals for egg activation in mammals. J Pharmacol Sci 100:545–552
Mogensen HL (1988) Exclusion of male mitochondria and plastids during syngamy in barley as a basis for maternal inheritance. Proc Natl Acad Sci USA 85:2594–2597
Mòl R, Filek M, Machackova I, Matthys-Rochon E (2004) Ethylene synthesis and auxin augmentation in pistil tissues are important for egg cell differentiation after pollination in maize. Plant and Cell Physiol 45:1396–1405
Morales-Ruiz T, Ortega-Galisteo AP, Ponferrada-Marın MI, Martínez-Macías MI, Ariza RR, Roldán-Arjona T (2006) DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc Natl Acad Sci USA 103:6853–6858
Müller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, Miller EL, O’Connor MB, Kingston RE, Simon JA (2002) Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111:197–208
Müller J, Kassis JA (2006) Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Curr Opin Genet Dev 16:476–84
Ngo QA, Moore JM, Baskar R, Grossniklaus U, Sundaresan V (2007) Arabidopsis GLAUCE promotes fertilization-independent endosperm development and expression of paternally inherited alleles. Development 134:4107–17
Ni DA, Wang LJ, Ding CH, Xu ZH (2001) Auxin distribution and transport during embryogenesis and seed germination of Arabidopsis. Cell Res 11:273–278
Nolan KE, Saeed NA, Rose RJ (2006) The stress kinase gene MtSK1 in Medicago truncatula with particular reference to somatic embryogenesis. Plant Cell Rep 25:711–722
Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A (2006) A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nature Genet 38:63–67
Ohad N, Margossian L, Hsu YC, Williams C, Repetti P, Fischer RL (1996) A mutation that allows endosperm development without fertilization. Proc Natl Acad Sci USA 93:5319–5324
Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Harada JJ, Goldberg RB, Fischer RL (1999) Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell 11:407–416
Peloquin SJ, Hougas RW (1959) Decapitation and genetic markers as related to haploidy in Solanum tuberosum. Eur Potato J 2:176–183
Quarin CL (1999) Effect of pollen source on endosperm formation and seed set in pseudogamous apomictic Paspalum notatum. Sex Plant Reprod 11:331–335
Ribnicky DM, Cohen JD, Hu WS, Cooke TJ (2002) An auxin surge following fertilization in carrots: a mechanism for regulating plant totipotency. Planta 214:505–509
Ringrose L, Paro R (2004) Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet 38:413–443
Röber FK, Gordillo GA, Gieger HH (2005) In vivo haploid induction in maize—Performance of new inducers and significance of double haploid line in hybrid breeding. Maydica 50:275–283
Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure J-E (2003) Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr Biol 13:432–436
Sarkar KR, Coe EH (1966) A genetic analysis of the origin of maternal haploids in maize. Genetics 54:453–464
Sørensen MB, Chaudhury AM, Robert H, Bacharel E, Berger F (2001) Polycomb group genes control pattern formation in plant seed. Curr Biol 11:277–281
Spielman M, Vinkenoog R, Scott RJ (2003) Genetic mechanisms of apomixis. Philos Trans R Soc London B 358:1095–1103
Springer PS, Holding DR, Groover A, Yordan C, Martienssen RA (2000) The essential Mcm7 protein PROLIFERA is localized to the nucleus of dividing cells during the G(1) phase and is required maternally for early Arabidopsis development. Development 127:1815–1822
Steinhardt RA, Eppel D (1974) Activation of sea urchin eggs by a calcium ionophore. Proc Natl Acad Sci USA 71:1915–19
Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 98:11806–11811
Tsunewaki K, Mukai Y (1990) Wheat haploids through the Salmon method. In: Bajaj YPS (ed) Wheat: biotechnology in agriculture and forestry. Vol 13. Springer, Heidelberg, pp 460–478
Tucker MR, Araujoc A-CG, Paecha NA, Hecht V, Schmidt EDL, Rossell J-B, de Vries SC, Koltunow AMG (2003) Sexual and apomictic reproduction in Hieracium subgenus pilosella are closely interrelated developmental pathways. Plant Cell 15:1524–1537
Uranga JA, Pedersen RA, Arechaga J (1996) Parthenogenetic activation of mouse oocytes using calcium ionophores and protein kinase C stimulators. Int J Dev Biol 40:515–19
Van Dijk PJ, Van Baarlen P, De Jong JH (2004) The occurrence of phenotypically complementary apomixis-recombinants in crosses between sexual and apomictic dandelions (Taraxacum officinale). Sex Plant Reprod 16:71–76
Vielle-Calzada J-P, Baskar R, Grossniklaus U (2000) Delayed activation of the paternal genome during seed development. Nature 404:91–94
Vielle-Calzada J-P, Baskar R, Grossniklaus U (2001) Seed development (Communication arising) Early paternal gene activity in Arabidopsis—response. Nature 414:710–710
Vielle-Calzada J-P, Thomas J, Spillane C, Coluccio A, Hoeppner MA, Grossniklaus U (1999) Maintenance of genomic imprinting at the Arabidopsis medea locus requires zygotic DDM1 activity. Genes Dev 13:2971–2982
Vinkenoog R, Spielmann M, Scott RJ (2001) Autonomous endosperm development in flowering plants: how to overcome the imprinting problem? Sex Plant Reprod 14:189–194
Weijers D, Geldner N, Offringa R Jürgens G (2001) Seed development (Communication arising): early paternal gene activity in Arabidopsis. Nature 414:709–710
Xiao W, Gehring M, Choi Y, Margossian L, Pu H, Harada JJ, Goldberg RB, Pennell RI, Fischer RL (2003) Imprinting of the MEA Polycomb gene is controlled by antagonism between MET1 methyltransferase and DME glycosylase. Dev Cell 5:891–901
Yadegari R, Kinoshita T, Lotan O, Cohen G, Katz A, Choi Y, Katz A, Nakashima K, Harada JJ, Goldberg RB, Fischer RL, Ohad N (2000) Mutations in the FIE and MEA genes that encode interacting Polycomb proteins cause parent-of-origin effects on seed development by distinct mechanisms. Plant Cell 12:2367–2382
Zenkteler M, Nitzsche W (1984) Wide hybridization experiments in cereals. Theor Appl Genet 68:311–315
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Dresselhaus.
Rights and permissions
About this article
Cite this article
Curtis, M.D., Grossniklaus, U. Molecular control of autonomous embryo and endosperm development. Sex Plant Reprod 21, 79–88 (2008). https://doi.org/10.1007/s00497-007-0061-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-007-0061-9