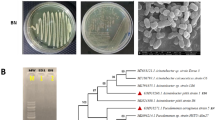
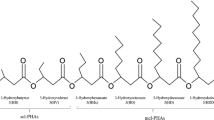
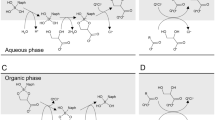
Abstract
Rhamnolipids (RLs) are anionic biosurfactants with great application potential. This study explored the possibility of producing RLs from cooking oil fume condensates (COFCs) collected from range hoods. A mutant of Pseudomonas aeruginosa AB93066 was obtained and used to produce RLs from COFCs as a substrate. RL yields in a 7-L fermenter reached 12.3 g/L, and MALDI–TOF MS showed that Rha2–C10–C10 and Rha–C10–C10 are the most abundant (39.6% and 26.4%, respectively) RL components. The critical micellar concentration (CMC) of the RLs was 45.0 mg/L and the surface tension of water decreased from 60.5 to 25.3 mN/m. Using six kinds of common hydrocarbons as indices, the emulsification coefficients of the RLs obtained were found to exceed 60%; in particular, the emulsification coefficient for benzene was 80.3%. COFCs provide an inexpensive alternative as a substrate for RL production, and the synthetic process is relatively harmless and economical.






Similar content being viewed by others
References
Shields PG, Xu GX, Blot WJ, Fraumeni JF Jr, Trivers GE, Pellizzari ED, Qu YH, Gao YT, Harris CC (1995) Mutagens from heated Chinese and U.S. cooking oils. J Natl Cancer Inst 87:836–841
Jiang X, Zhu S, Wu Y, Huai H (2009) The effects of cooking oil fume condensates (COFCs) on the vegetative growth of Salvinia natans (L.) All. J Hazard Mater 172:240–246
Zhu X, Wang K, Zhu J, Koga M (2001) Analysis of cooking oil fumes by ultraviolet spectrometry and gas chromatography-mass spectrometry. J Agric Food Chem 49:4790–4794
Wu M, Che W, Zhang Z (2008) Enhanced sensitivity to DNA damage induced by cooking oil fumes in human OGG1 deficient cells. Environ Mol Mutagen 49:265–275
Chiang TA, Wu PF, Wang LF, Lee H, Lee CH, Ko YC (1997) Mutagenicty and polycyclic aromatic hydrocarbon content of fumes from heated cooking oils produced in Taiwan. Food Chem Toxicol 37:125–134
Kreuzer M, Heinrich J, Kreienbrock L, Rosario AS, Gerken M, Wichmann HE (2002) Risk factors for lung cancer among nonsmoking women. Int J Cancer 100:706–713
Ko YC, Cheng SC, Lee CH, Huang JJ, Huang MS, Kao EL, Wang HZ, Lin HJ (2000) Chinese food cooking and lung cancer in women nonsmokers. Am J Epidemiol 151:140–147
Metayer C, Wang ZY, Klerinerman RA, Wang LD, Brennner AV, Cui HX, Cao JS, Lubin JH (2002) Cooking oil fumes and risk of lung cancer in women in rural Gansu, China. Lung Cancer 35:111–117
Feng SD, Ling HY, Chen F (2003) Meta analysis of female lung cancer associated with cooking oil fume. J Environ Health 20:353–354
Maier RM, Soberón-Chávez G (2000) Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications. Appl Microbiol Biotechnol 54:625–633
Reis RS, Pereira AG, Neves BC, Freire DM (2011) Gene regulation of rhamnolipid production in Pseudomonas aeruginosa–a review. Bioresour Technol 102:6377–6384
Sachdev DPA, Cameotra SSB (2013) Biosurfactants in agriculture. Appl Microbiol Biotechnol 97:1005–1016
Gong ZJ, Peng YF, Wang QH (2015) Rhamnolipid production, characterization and fermentation scale-up by Pseudomonas aeruginosa with plant oils. Biotechnol Lett 37:2033–2038
Zhu L, Yang X, Xue C, Chen Y, Qu L, Lu W (2012) Enhanced rhamnolipids production by Pseudomonas aeruginosa based on a pH stage-controlled fed-batch fermentation process. Bioresour Technol 117:208–213
Müller MM, Hörmann B, Syldatk C, Hausmann R (2010) Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Appl Microbiol Biotechnol 87:167–174
Wu J, Zhang J, Wang P, Zhu L, Gao M, Zheng Z, Zhan X (2017) Production of rhamnolipids by semi-solid-state fermentation with Pseudomonas aeruginosa RG18 for heavy metal desorption. Bioprocess Biosyst Eng 40:1611–1619
Abalos A, Maximo F, Manresa MA, Bastida J (2002) Utilization of response surface methodology to optimize the culture media for the production of rhamnolipids by Pseudomonas aeruginosa AT10. J Chem Technol Biotechnol 77:777–784
Lan G, Fan Q, Liu Y, Chen C, Li G, Liu Y, Yin X (2015) Rhamnolipid production from waste cooking oil using Pseudomonas SWP-4. Biochem Eng J 101:44–54
Benincasa M, Contiero J, Manresa MA, Moraes IO (2002) Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J Food Eng 54:283–288
Rebello S, Asok AK, Joseph SV, Joseph BV, Jose L, Mundayoor SMSJ (2013) Bioconversion of sodium dodecyl sulphate to rhamnolipid by Pseudomonas aeruginosa: a novel and cost-effective production strategy. Appl Biochem Biotechnol 169:418–430
Henkel M, Müller MM, Kügler JH, Lovaglio RB, Contiero J, Syldatk C, Hausmann R (2012) Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process Biochem 47:1207–1219
Zhang L, Veres-Schalnat TA, Somogyi A, Pemberton JE, Maier RM (2012) Fatty acid cosubstrates provide β-oxidation precursors for rhamnolipid biosynthesis in Pseudomonas aeruginosa, as evidenced by isotope tracing and gene expression assays. Appl Environ Microbiol 78:8611–8622
Abo-Amer AE (2007) Involvement of chromosomally-encoded genes in malathion utilization by Pseudomonas aeruginosa AA112. Acta Microbiol Immunol Hung 54:261–277
Soberon-Chavez G (ed) (2011) Biosurfactants, Microbiology Monographs, vol 20. Springer-Verlag, Berlin, pp 17–21
Oliveira FJS, Vazquez L, de Campos NP, de França FP (2008) Production of rhamnolipid by a Pseudomonas alcaligenes strain. Process Biochem 44:383–389
Rahman KS, Rahman TJ, McClean S, Marchant R, Banat IM (2002) Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnol Prog 18:1277–1281
Price NP, Ray KJ, Vermillion K, Kuo TM (2009) MALDI-TOF mass spectrometry of naturally occurring mixtures of monorhamnolipids and dirhamnolipids. Carbohydr Res 344:204–209
Santana-Filho APD, Noleto GR, Gorin PAJ, Souza LMD, Iacomini M, Sassaki GL (2012) GC-MS detection and quantification of lipopolysaccharides in polysaccharides through 3-O-acetyl fatty acid methyl esters. Carbohydr Poly 87:2730–2734
Abdel-Mawgoud AM, Lépine F, Déziel E (2014) A stereospecific pathway diverts β-oxidation intermediates to the biosynthesis of rhamnolipid biosurfactants. Chem Biol 21:156–164
Zhang L, Pemberton JE, Maier RM (2014) Effect of fatty acid substrate chain length on Pseudomonas aeruginosa ATCC 9027 monorhamnolipid yield and congener distribution. Process Biochem 49:989–995
Kostal J, Suchanek M, Klierova H, Demnerova K, Kralova B, McBeth DL (1998) Pseudomonas C12B, an SDS degrading strain, harbours a plasmid coding for degradation of medium chain length n-alkanes. Int Biodeterior Biodegrad 42:221–228
Abdel-Mawgoud AM, Hausmann R, Lepine F, Muller MM, Deziel E (2011) Biosurfactants—from genes to applications. Springer, Berlin, pp 14–55
Zhu S, Wu Y, Wen G, Bai W, Hao Z, Huai H (2011) Effects of main chemical compounds in cooking oil fume condensates (COFCs) on growth of Salvinia natans (L.) All.: I. Dodecane. Nat Env Poll Tech 10:7–13
Zhu S, Wu Y, Wen G, Bai W, Hao Z, Huai H (2011) Effects of main chemical compounds in cooking oil fume condensates (COFCs) on growth of Salvinia natans (L.) All.: II. Hexadecane. Nat Env Poll Tech 10:331–336
Haba E, Espuny MJ, Busquets M, Manresa A (2000) Screening and production of rhamnolipids by Pseudomonas aeruginosa 47T2 NCIB 40044 from waste frying oils. J Appl Microbiol 88:379–387
Nitschke M, Costa SG, Haddad R, Gonçalves LA, Eberlin MN, Contiero J (2005) Oil wastes as unconventional substrates for rhamnolipid biosurfactant production by Pseudomonas aeruginosa LBI. Biotechnol Prog 21:1562–1566
Perfumo A, Rudden M, Smyth TJ, Marchant R, Stevenson PS, Parry NJ, Banat IM (2013) Rhamnolipids are conserved biosurfactants molecules: implications for their biotechnological potential. Appl Microbiol Biotechnol 97:7297–7306
Raza ZA, Khan MS, Khalid ZM, Rehman A (2006) Production kinetics and tensioactive characteristics of biosurfactant from a Pseudomonas aeruginosa mutant grown on waste frying oils. Biotechnol Lett 28:1623–1631
Kłosowska-Chomiczewska IE, Mędrzycka K, Hallmann E, Karpenko E, Pokynbroda T, Macierzanka A, Jungnickel C (2017) Rhamnolipid CMC prediction. J Colloid Interface Sci 488:10–19
Abalos A, Pinazo A, Infante MR, Casals M, García F, Manresa A (2001) Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir 17:1367–1371
Benincasa M, Accorsini FR (2008) Pseudomonas aeruginosa LBI production as an integrated process using the wastes from sunflower-oil refining as a substrate. Bioresour Technol 99:3843–3849
Raza ZA, Rehman A, Khan MS, Khalid ZM (2007) Improved production of biosurfactant by a Pseudomonas aeruginosa mutant using vegetable oil refinery wastes. Biodegradation 18:115–121
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21778022), the Program of Introducing Talents of Discipline to Universities (No. 111-2-06), the Fundamental Research Funds for the Central Universities (No. JUSRP51632A), and the National First-class Discipline Program of Light Industry Technology and Engineering (No. LITE2018-17).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, J., Zhang, J., Zhang, H. et al. Recycling of cooking oil fume condensate for the production of rhamnolipids by Pseudomonas aeruginosa WB505. Bioprocess Biosyst Eng 42, 777–784 (2019). https://doi.org/10.1007/s00449-019-02081-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02081-1