Abstract
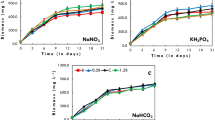
Culture conditions for the mass production of three green algae, Chlorella sp., Dunaliella salina DCCBC2 and Dunaliella sp., were optimized using a response surface methodology (RSM). A central composite design was applied to investigate the effects of initial pH, nitrogen and phosphate concentrations on the cultivation of microalgae. The optimal growth conditions estimated from the design are as follows: Chlorella sp. (initial pH 7.2, ammonium 17 mM, phosphate 1.2 mM), D. salina DCCBC2 (initial pH 8.0, nitrate 3.3 mM, phosphate 0.0375 mM) and Dunaliella sp. (initial pH 8.0, nitrate 3.7 mM, phosphate 0.17 mM). Culturing the microalgae with the optimized conditions confirmed that the maximum growth rates were attained for these parameters. The optimum CO2 concentrations of Chlorella sp., D. salina DCCBC2 and Dunaliella sp. were 1.0, 3.0 and 1.0% (v/v), respectively. The specific growth rates (μ) of Chlorella sp., D. salina DCCBC2 and Dunaliella sp. were 0.58, 0.78 and 0.56 day−1, respectively, and the biomass productivities were 0.28, 0.54 and 0.30 g dry cell wt l−1 day−1, respectively. The CO2 fixation rates of Chlorella sp., D. salina DCCBC2 and Dunaliella sp. were 42.8, 90.9 and 45.5 mg l−1 day−1, respectively. Mixotrophic cultivation of Chlorella sp. with glucose increased biomass productivity from 0.28 to 0.51 g dry cell wt l−1 day−1. However, D. salina DCCBC2 and Dunaliella sp. were not stimulated by several organic compounds tested.





Similar content being viewed by others
References
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Leon-Banares R, Gonzalez-Ballester D, Galvan A, Fernandez E (2004) Transgenic as green cell-factories. Trends Biotechnol 22:45–52
Koh LP, Ghazoul J (2008) Biofuels, biodiversity, and people: Understanding the conflicts and finding opportunities. Biol Conserv 141:2450–2460
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: A review. Renew Sustain Energ Rev 14:217–232
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biot 65:635–648
Field CB (1998) Primary production of the biosphere: integrating terrestrial and oceanic compounds. Science 281:237–240
Weissman JC, Tillett DT (1992) Design and operation of an outdoor microalgae test facility: Large-scale system results. In: Brown LM, Sprague S (eds) Aquatic species project report. National Renewable Energy Laboratory, Golden, pp 32–56
Kumar AS, Ergas XY, Sahu A, Zhang Q, Dewulf J, Malcata FX, Langenhove H (2010) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380
Myers RH, Montgomery DC (2002) Response surface methodology; process and product optimization using designed experiments, 2nd edn. Wiley, New York
Kim H, Eom HJ, Lee JS, Han JS, Han NS (2004) Statistical optimization of medium composition for growth of Leuconostoc citreum. Biotechnol Bioproc E 9:278–284
Polle JE, Struwe L, Jin E (2008) Identification and characterization of a new strain of the unicellular green alga Dunaliella salina (Teod.) from Korea. J Microbiol Biotechnol 18:821–827
Park S, Polle JE, Mellis A, Lee TK, Jin E (2006) Up-regulation of photoprotection and PSII-repair gene expression by irradiance in the unicellular green alga Dunaliella salina. Mar Biotechnol 8:120–128
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. PNAS 54:1665–1669
Dora S (1976) Determination of ammonia and kjeldahl nitrogen by indophenols method. Water Res 10:31–36
Towns TG (1986) Determination of aqueous phosphate by ascorbic acid reduction of phosphomolybdic acid. Anal Chem 58:223–229
Gonzalez LE, Canizars RO, Baena S (1997) Efficiency of ammonia and phosphorus removal from a Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresource Technol 60:259–262
Chevalier P, Noue J (1985) Wastewater nutrient removal with microalgae immobilized in Carrageenan. Enzyme Microb Technol 7:621–624
Ryu HJ, Oh KK, Kim YS (2009) Optimization of the influential factors for the improvement of CO2 utilization efficiency and CO2 mass transfer rate. J Ind Eng Chem 15:471–475
de Greque MM, Costa JAV (2007) Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol Lett 29:1349–1352
Francisco EC, Neves DB, Jacob-Lopesb E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Ceron Garcia MC, Garcia Camacho F, Sanchez Miron A, Fernandez Sevilla JM, Chisti Y, Molina Grima E (2006) Mixotrophic production of marine microalga Phaeodactylum tricornutum on various carbon sources. J Microbiol Biotechnol 16:689–694
Ukeles R, Rose WE (1976) Observation on organic carbon utilization by photosynthetic marine microalgae. Mar Biol 37:11–28
Acknowledgments
This work was supported by the grant of New & Renewable Energy Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) funded by the Ministry of Knowledge Economy, Korea (20103020090020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, W., Park, J.M., Gim, G.H. et al. Optimization of culture conditions and comparison of biomass productivity of three green algae. Bioprocess Biosyst Eng 35, 19–27 (2012). https://doi.org/10.1007/s00449-011-0612-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0612-1